Table of Contents
The Central Board of Secondary Education has successfully conducted the CBSE Class 12th Chemistry Board exam for all the Class 12th Science Stream students today i.e., February 27, 2025 [Thursday]. We have shared the set-wise CBSE 12th Chemistry Question Paper 2025 here. As the final revision continues, students are focusing on their class 12 Chemistry sample papers, along with the previous year’s papers for their end-time preparation. These papers will provide an idea of the paper pattern and the type of questions asked. For more updates regarding the CBSE Class 12th Chemistry Question Paper 2025, students must go through the complete article below.
CBSE Class 12th Chemistry Question Paper 2025 Out
The CBSE Class 12th Chemistry Exam 2025 was successfully held in traditional offline pen-and-paper mode at various exam centers across the country. Students were allotted a time duration of 3 hours to complete the exam whereas an extra 15 minutes will be given for reading the question paper.
Chemistry is always a challenging subject for students due to its vast scope and both descriptive and mathematical nature. The CBSE class 12th Chemistry Question Paper 2025 had a similar paper pattern to the CBSE 12th Sample papers. After the successful conduction of the CBSE Class 12 Chemistry Exam 2025 students can check the complete Class 12 Chemistry answer key 2025 here.
Class 12 Chemistry Question Paper 2025
The Chemistry Question paper includes questions from different sections like physical, organic, and inorganic chemistry. The total marks for the CBSE class 12th Chemistry exam is for a total of 100 marks out of which the theory paper was 70 marks while the rest 30 marks were provided for the Chemistry Practical exam 2024-25.
| CBSE Class 12th Chemistry Question Paper 2025 | |
| Conducting Body | Central Board of Secondary Education |
| Name of Examination | CBSE Class 12 Chemistry Exam 2024-25 |
| Category | Question paper |
| Status | Released |
| CBSE class 12 Chemistry Exam Date 2025 | February 27, 2025 |
| Exam Timing | 10:30 am to 01:30 pm |
| Mode of Examination | Online mode |
| Total Marks | 100 |
| Negative Marking | There is no negative marking |
CBSE Class 12 Chemistry Exam Pattern 2025
The CBSE Class 12 Chemistry Questions Paper 2024-25 was completely designed based on the lasted CBSE board exam pattern. CBSE 12 Chemistry Question Paper 2025 consisted of a total of 33 questions which will be further divided into 5 sections with a good amount of internal choices. The complete exam pattern that has been followed is as follows-
| CBSE Class 12 Chemistry Exam Pattern 2025 | ||
| Section | Types of Question | Marks |
| Section A | Multiple Choice Questions | 1 Mark each |
| Section B | Very Short Answer Type Questions | 2 Marks each |
| Section C | Short Answer Type Questions | 3 marks each |
| Section D | Case-Based Questions | 4 marks each |
| Section E | Long Answer Type Questions | 5 marks each |
CBSE Class 12th Chemistry Question Paper 2025 PDF Download
As the CBSE Class 12th Chemistry Exam for the academic session 2024-25 is successfully held, we have shared direct links to download the CBSE Class 12 Chemistry Question Paper 2025 in the table mentioned below. These question papers will help students both after and before the exams. After the completion of the exam, students will be able to review their answers with the help of these set-wise question papers and answer keys. These papers will also benefit those students who are going to appear for the 2025-26 session board exams, as they will help them get an idea of the exam pattern. The direct links for set-wise CBSE Class 12 Chemistry Question Paper 2025 have been shared here.
| CBSE Class 12 Chemistry Question Paper 2025 PDF Download | |
| Sets | Download Link |
| CBSE Class 12 Chemistry Question Paper 2025 Set 1 | Click Here |
| CBSE Class 12 Chemistry Question Paper 2025 Set 2 | Click Here |
| CBSE Class 12 Chemistry Question Paper 2025 Set 3 | Click Here |
CBSE Class 12 Chemistry Question Paper 2025 With Answers – Set 1
Section A
Q1. The charge required for the reduction of 1 mol of MnO¯4 to MnO₂ is
(A)1 F
(B) 3 F
(D) 5F
(D) 6 F
Answer: (B) 3 F
Q2. Which among the following is a false statement?
(A) Rate of zero order reaction is independent of initial concentration of reactant.
(B) Half-life of a zero order reaction is inversely proportional to the rate constant.
(C) Molecularity of a reaction may be zero.
(D) For a first-order reaction, t1/2= 0.693/k.
Answer: (C) Molecularity of a reaction may be zero.
Q3. The number of molecules that react with each other in an elementary reaction is a measure of the
(A) the activation energy of the reaction
(B) stoichiometry of the reaction
(C) the molecularity of the reaction
(D) order of the reaction
Answer: (C) molecularity of the reaction.
Q4. The element having [Ar]3d104s2 electronic configuration is
(A) Cu
(B) Zn
(C) Cr
(D) Mn
Answer: (B) Zn
Q5. The complex ions [Co(NH3)5(NO2)]2+ and [Co(NH3)5(ONO)]2+ are called
(A) Ionization isomers
(B) Linkage isomers
(C) Co-ordination isomers
(D) Geometrical isomers
Answer: (B) Linkage isomers.
Q6. The diamagnetic species is:
(A) [Ni(CN)4]2-
(B) [NiCl4]2−
(C) [Fe(CN)6]3−
(D) [CoF6]3−
Answer: (A) [Ni(CN)4]2-
Q7. Which is the correct IUPAC name for the given compound?
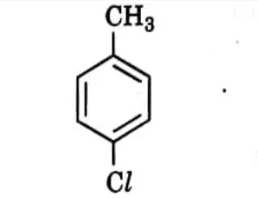
(A) Methylchlorobenzene
(B) Toluene
(C) 1-Chloro-4-Methylbenzene
(D) 1-Methyl-4-Chlorobenzene
Answer: (C) 1-Chloro-4-Methylbenzene
8. What will be formed after the oxidation reaction of secondary alcohol with chromic anhydride (CrO3)?
(A) Aldehyde
(B) Ketone
(C) Carboxylic acid
(D) Ester
Answer: (B) Ketone.
Q9. The conversion of phenol to salicylic acid can be accomplished by\
(A) Reimer-Tiemann reaction
(B) Friedel-Crafts reaction
(C) Kolbe reaction
(D) Coupling reaction
Answer: (C) Kolbe reaction
Q10. Which of the following is/are examples of denaturation of protein?
(A) Coagulation of egg white
(B) Curdling of milk
(C) Clotting of blood
(D) Both (A) and (B)
Answer: (D) Both (A) and (B)
Q11. Nucleotides are joined together by
(A) Glycosidic linkage
(B) Peptide linkage
(C) Hydrogen bonding
(D) Phosphodiester linkage
Answer: (D) Phosphodiester linkage
12. Scurvy is caused due to deficiency of
(A) Vitamin B1
(B) Vitamin B2
(C) Ascorbic acid
(D) Glutamic acid
Answer: (C) Ascorbic acid
For questions numbers 13 to 16, two statements are given – one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C), and (D) as given below:
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
Q13. Assertion (A): In a first-order reaction, if the concentration of the reactant is doubled, its half-life is also doubled.
Reason (R): The half-life of a reaction does not depend upon the initial concentration of the reactant in a first-order reaction.
Answer: (D) Assertion (A) is false, but Reason (R) is true.
Q14. Assertion (A): Cu cannot liberate H2 in reaction with dilute mineral acids.
Reason (R): Cu has a positive electrode potential.
Answer: (A) Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of the Assertion (A).
Q15. Assertion (A): Aromatic primary amines cannot be prepared by Gabriel Phthalimide synthesis.
Reason (R): Aryl halides do not undergo nucleophilic substitution reaction with the anion formed by phthalimide.
Answer: (A) Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of the Assertion (A).
Q16. Assertion (A): Vitamin D cannot be stored in our body.
Reason (R): Vitamin D is a fat-soluble vitamin and is not excreted from the body in urine.
Answer: (D) Assertion (A) is false, but Reason (R) is true.





 CUET English Previous Year Question Pape...
CUET English Previous Year Question Pape...
 CBSE Class 12 Accountancy Answer Key 202...
CBSE Class 12 Accountancy Answer Key 202...
 CBSE Class 12th Political Science Questi...
CBSE Class 12th Political Science Questi...