Table of Contents
What methods can we use to separate mixtures? Separation processes are ways to divide mixtures into their individual components. Some common methods include filtration, where a filter is used to separate solid particles from liquids; distillation, which separates liquids based on their boiling points; and evaporation, where a liquid turns into vapor, leaving solid residues behind. Another method is magnetic separation, which uses magnets to pull out magnetic materials from mixtures. In the article below we have discussed more about the process of sedimentation, decantation, and filtration.
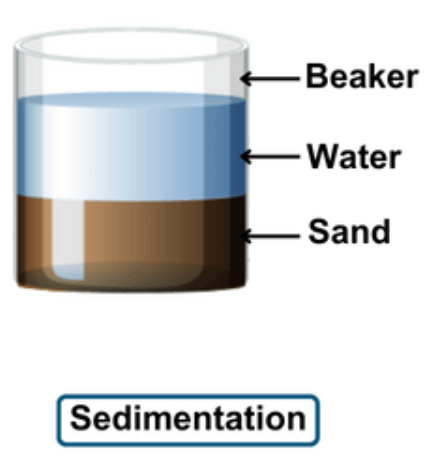
What is Sedimentation?
Sedimentation is a natural process where solid particles, like sand or silt, settle at the bottom of a liquid. This usually happens in rivers, lakes, or even in containers of water. When water flows slowly or stands still, heavier particles in it sink down due to gravity, forming layers of sediment. This process helps separate solid impurities from water, making it clearer. Sedimentation is used in water treatment plants to remove dirt and other particles from water before it is filtered and purified. It’s also common in nature, forming layers of soil and rocks over many years.
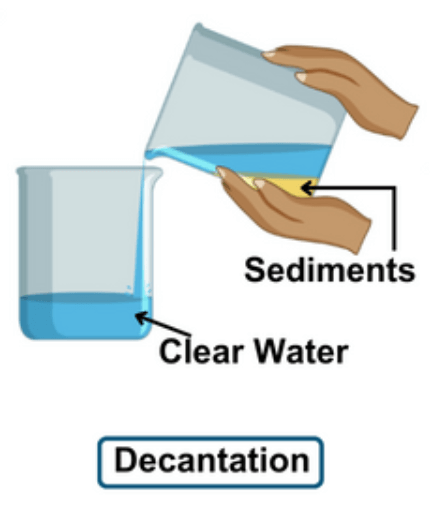
Define Decantation
Decantation is a process used to separate mixtures. It involves pouring off a liquid from a solid that has settled at the bottom of a container. For example, when sand and water are mixed, the sand sinks to the bottom. To decant, you carefully tilt the container and pour the water into another one, leaving the sand behind. This method is useful for separating liquids from solids or two liquids that don’t mix well, like oil and water. Decantation is simple and doesn’t require any special equipment, making it easy to do at home or in a lab.
What is Filtration?
Filtration is a process used to separate solids from liquids or gases. It works by passing a mixture through a filter, which is a material with tiny holes. These holes allow only smaller particles, like liquids or gases, to pass through, while larger particles are trapped.
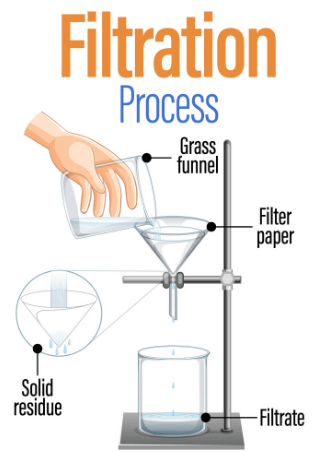
For example, when you make coffee, you pour hot water through a coffee filter. The water goes through the filter, carrying the flavor, but the coffee grounds are left behind. Filtration is commonly used in many places, like in water treatment plants to clean drinking water, in laboratories for experiments, and in cooking. It helps remove unwanted materials and keeps things clean and safe.
Difference Between Sedimentation, Decantation and Filtration
| Difference Between Sedimentation, Decantation and Filtration | |||
| Aspects | Sedimentation | Decantation | Filtration |
| Definition | The process of allowing suspended particles to settle at the bottom of a liquid due to gravity. | The process of carefully pouring off the liquid from the settled liquid from the settled solid without disturbing the sediment. | The process of separating solids from liquids or gases using a filter medium that allows only fluid to pass through. |
| Purpose | To separate solid particles from a liquid by letting them settle. | To remove the clear liquid from the sediment after sedimentation. | To separate smaller particles from a mixture, typically to purify liquids. |
| Mechanism | Relies on the force of gravity to allow denser particles to sink. | Involves tilting the container to pour off the liquid layer. | Uses a barrier (filter) that traps solid particles while allowing liquids or gases to pass. |
| Apparatus Used | Typically involves a container like a beaker or sedimentation tank. | Requires the same container as sedimentation, often with a pouring technique. | Requires a filter paper or membrane placed in a funnel or filter apparatus. |
| Time required | May take several minutes to hours, depending on particle size and density. | Usually quick, as it involves simply pouring out the liquid. | Can be immediate or take longer based on the filter type and viscosity of the liquid. |
| Examples | Clarify muddy water by letting it stand so the soil settles. | Pouring off clear water from a container after sedimentation. | Brewing coffee using a filter to separate grounds from liquid coffee. |
| Effect on Solid | Solid particles settle at the bottom, creating a layer of sediment. | Sediment remains undisturbed at the bottom while the liquid is removed. | Solid particles are retained on the filter while the liquid passes through. |
| Efficiency | Efficient for separating large, heavier particles but may not work well for very fine particles. | Effective for separating liquid from settled solids, but relies on sedimentation first. | Highly efficient for separating fine solids from liquids or gases. |
| Application | Used in water treatment, mining, and environmental science. | Commonly used in laboratories and kitchen practices (like separating oil from water). | Widely used in laboratories, chemical industries, and everyday processes (like making juice). |
Different Uses of Sedimentation, Decantation and Filtration
Sedimentation, decantation, and filtration are techniques used in various fields, including chemistry, environmental science, and industry. These techniques are essential for ensuring the purity and quality of various substances across multiple applications, from environmental management to industrial processes. Here are their different uses:
1. Sedimentation
- Water Treatment: Used to remove suspended solids from water by allowing them to settle at the bottom of a tank.
- Mining and Mineral Processing: Helps in separating minerals from ores by allowing heavier particles to settle out.
- Soil Science: Used to analyze soil texture by determining the proportions of sand, silt, and clay.
- Biological Processes: In wastewater treatment, sedimentation allows solid waste to settle before further processing.
2. Decantation
- Separation of Liquids: Used to separate liquids that do not mix (immiscible) or to remove liquid from settled solids after sedimentation.
- Cooking: In culinary processes, such as separating oil from water or broth after boiling.
- Laboratory Procedures: Used in chemical experiments to separate the supernatant liquid from precipitates.
- Wine Production: Helps in clarifying wine by removing sediment after fermentation.
3. Filtration
- Water Purification: Removes contaminants and impurities from drinking water and industrial wastewater.
- Laboratory Applications: Separates solids from liquids in chemical reactions or analyses.
- Air Quality Control: Uses filters to trap particles and pollutants in HVAC systems and air purifiers.
- Food Industry: Used to clarify juices, beer, and other beverages by removing solids.





 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 Reactivity Series of Metals, Charts, Fea...
Reactivity Series of Metals, Charts, Fea...









