Table of Contents
Saponification is a topic from the class 10th Chemistry chapter “Carbon and its Compounds”. It is a chemical reaction where fats or oils are converted into soap and glycerin. This happens when fats or oils react with a strong base like sodium hydroxide (caustic soda) or potassium hydroxide. The base breaks down the fat molecules into fatty acids and glycerin. The fatty acids then react with the base to form soap. This process is crucial in making soap, which helps clean by trapping dirt and grease so they can be washed away. For more information regarding the process of saponification go through the complete article below.
What is Saponification?
Saponification is a chemical process that creates soap. It happens when fat or oil reacts with a strong base, such as sodium hydroxide (lye). During this reaction, the fat or oil molecules break down into two parts: glycerol (a type of alcohol) and soap. The soap formed is what we use for cleaning, while glycerol is often used in skincare products.
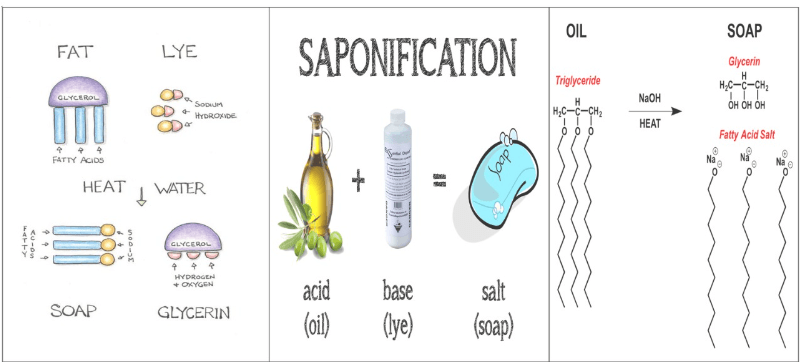
The process requires heat to work effectively. Saponification is essential because it’s the traditional method for making soap, using natural fats and oils. The result can be solid or liquid soap, depending on the specific ingredients and conditions used in the process. This simple reaction has been used for centuries to create everyday soaps.
In simple terms, saponification is the process of making soap by combining fats or oils with a strong base like NaOH, resulting in soap and glycerol.
Saponification Reaction
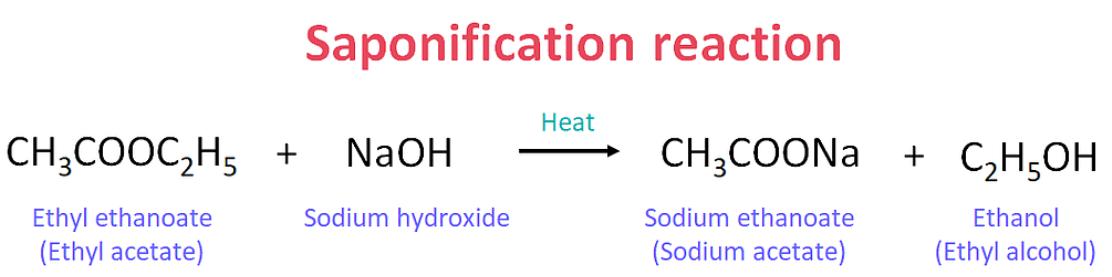
The saponification reaction is a chemical process that happens when fats or oils react with a strong base like sodium hydroxide (NaOH). When these substances mix, they produce soap and glycerol (a type of alcohol). The fats or oils are made up of molecules called triglycerides, which break down into soap and glycerol during the reaction. The soap formed helps clean by breaking down grease and dirt, making it easier to wash away with water. This reaction is important in making soaps we use every day. The term “saponification” comes from the Latin word “sapo,” which means soap. The Saponification Reaction can be written as:
| Easter + Base → Alcohol + Soap |
Step-Wise Mechanism of Saponification
Saponification is the chemical process used to make soap from fats or oils and an alkali. This process is essential in the production of traditional soaps and highlights the chemical transformation of fats and oils into useful cleaning products. Here we have discussed the step-by-step breakdown of the mechanism:
Step 1. Hydrolysis of Ester Bonds:
- Starting Materials: Triglycerides (fats/oils) and a strong base, typically sodium hydroxide (NaOH) or potassium hydroxide (KOH).
- Reaction: The triglyceride, which is an ester made up of glycerol and three fatty acid chains, reacts with the alkali.
- Mechanism: The hydroxide ion (OH-) from the alkali attacks the carbonyl carbon of the ester bond in the triglyceride.
- Result: This leads to the breaking of the ester bond, releasing a fatty acid salt (soap) and glycerol.
Step 2. Formation of Soap:
- Reaction: The free fatty acids formed in the first step react with the alkali.
- Mechanism: The alkali (e.g., NaOH) donates its sodium ion (Na+), which associates with the fatty acid to form a sodium salt of the fatty acid, commonly known as soap.
- Result: The long-chain fatty acid salt, which is the soap, has both hydrophobic (water-repelling) and hydrophilic (water-attracting) properties, enabling it to emulsify fats and oils in water.
Step 3. Separation of Glycerol:
- Reaction: Glycerol, a byproduct of the reaction, is formed alongside soap.
- Process: Glycerol, being more soluble in water, is separated from the soap by washing with water.
- Result: The final soap product is obtained as a solid mass after cooling and hardening, while glycerol can be extracted from the aqueous layer.
Step 4. Purification of Soap:
- Process: The crude soap is often purified by boiling with water and then adding salt (common salt or sodium chloride).
- Mechanism: The addition of salt helps to precipitate the soap, separating it from impurities and excess water.
- Result: After cooling, the purified soap solidifies and can be molded into bars or other shapes.
Summary:
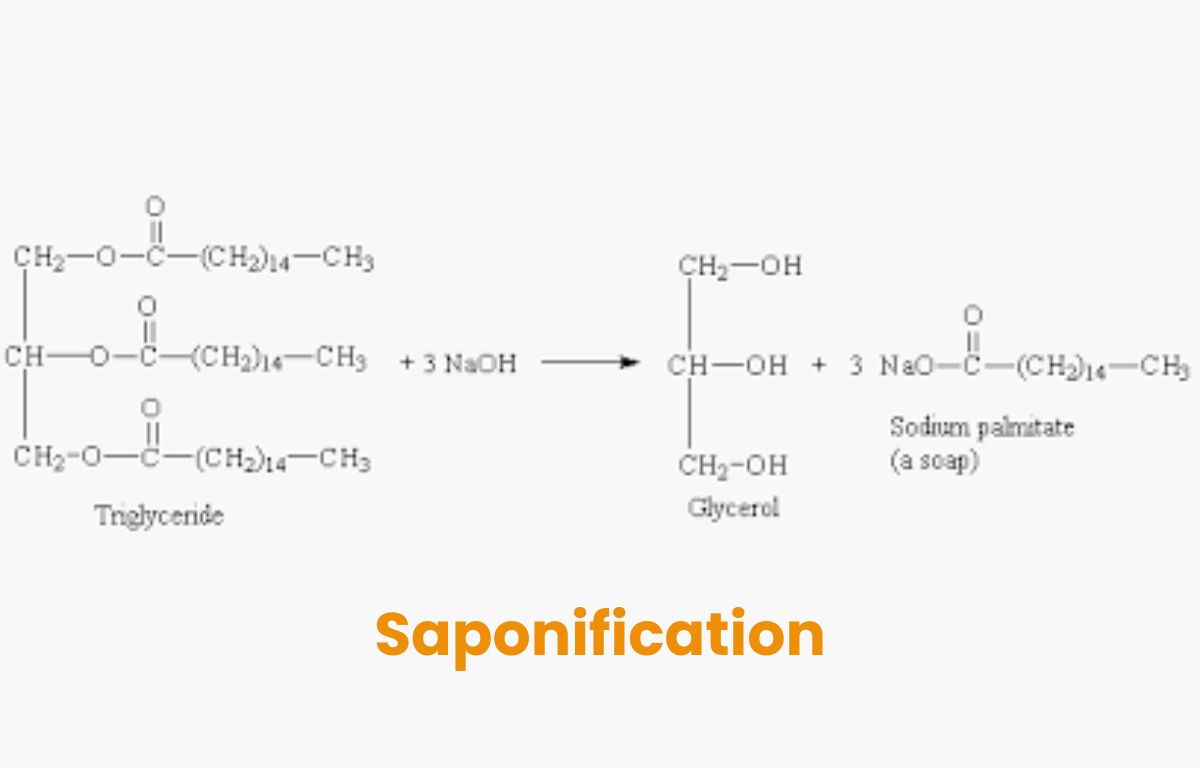
The overall saponification reaction can be simplified as:
| Fat (Triglyceride) + Alkali (NaOH or KOH) → Soap (Sodium or Potassium Salt of Fatty Acid) + Glycerol |
Effects of Saponification
Saponification is a chemical reaction that produces soap from fats or oils and a base, usually sodium hydroxide (NaOH). In summary, the effect of saponification is the production of soap and glycerol, both of which have useful properties, especially in cleaning and personal care products. The reaction also produces a basic substance, which is important for the soap’s ability to clean. Here’s a simple explanation of the effects of saponification:
- Formation of Soap: The primary effect of saponification is the production of soap. When fats or oils react with sodium hydroxide, the triglycerides (the main components of fats and oils) break down into glycerol and soap.
- Release of Glycerol: Glycerol, also known as glycerin, is a byproduct of the saponification process. It is a valuable substance used in many products, including cosmetics and pharmaceuticals.
- Cleaning Properties: The soap produced through saponification has cleaning properties because it can emulsify fats and oils, allowing them to be washed away with water. This is why soap is effective in cleaning greasy or oily substances.
- pH Level: The soap produced through saponification is usually basic, with a pH higher than 7. This basicity is due to the presence of leftover sodium hydroxide that might not have fully reacted.
- Hardness of Soap: The type of fat or oil used in saponification affects the hardness of the soap. For instance, animal fats tend to produce harder soaps, while vegetable oils like olive oil produce softer soaps.
- Environmental Impact: Saponification is an environmentally friendly process, as the products (soap and glycerol) are biodegradable. This means they can break down naturally without harming the environment.
Examples of Saponification
Below we have discussed a few examples of saponification. These examples illustrate how saponification is utilized in both industrial and everyday applications.
- Soap Making: In traditional soap making, saponification is used to convert animal fats or vegetable oils into soap. For instance, if you mix olive oil with sodium hydroxide (lye), you get olive oil soap and glycerol.
- Homemade Cleaning Products: Many homemade cleaning products use saponification. For example, a mixture of coconut oil and lye can be used to make a basic cleaning soap.
- Biodiesel Production: Saponification can occur in the production of biodiesel. If fats or oils react with a strong base, they form soap as a byproduct. This can be a problem in the biodiesel industry, where unwanted soap formation needs to be minimized.




 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...