Table of Contents
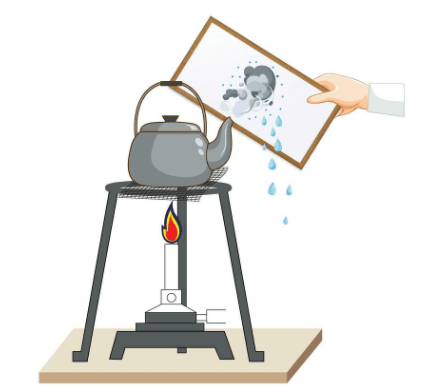
Have you ever wondered what happens when water disappears from a wet surface? This is called evaporation. Evaporation happens when the sun heats up water, turning it into water vapor, which is an invisible gas. The vapor then rises into the air. This process can happen with water in lakes, puddles, or even on our skin when we sweat. When we’re hot, our bodies produce sweat to cool us down. As the sweat evaporates from our skin, it takes away heat, making us feel cooler. So, evaporation helps move water around and even keeps us comfortable on hot days!
What is Evaporation?
Evaporation a the process by which a liquid, like water, changes into a gas, usually due to heat. When the sun shines or something is warm, it gives energy to the water molecules. This energy makes the molecules move faster and escape into the air as tiny particles or vapors. For example, if you leave a bowl of water outside on a sunny day, you’ll notice the water slowly disappears – that’s evaporation. It is a natural process that happens in rivers, lakes, oceans, and even on your skin when you sweat.
Steps Wise Process of Evaporation
Evaporation is a natural process that plays a significant role in the water cycle and helps regulate temperature and moisture in the environment. Below we have discussed the complete stepwise process of evaporation:
Step 1: Introduction to Evaporation
- Definition: Evaporation is the process by which a liquid changes into a gas. In this process, molecules in the liquid state gain enough energy to break free from the surface and turn into vapor.
- Examples: Water turning into water vapor, like when puddles dry up after rain, or when wet clothes dry in the sun.
Step 2: Molecule Movement in a Liquid
- In any liquid, molecules are constantly moving and have varying levels of energy.
- Some molecules move faster than others, and those near the surface of the liquid are more likely to escape into the air if they gain enough energy.
Step 3: Energy Transfer and Temperature
- Heat Energy: When heat is applied to a liquid (such as from sunlight), the molecules absorb this energy.
- Temperature and Movement: As temperature rises, the molecules move faster, increasing their kinetic energy. Molecules with higher energy are more likely to escape into the air.

Step 4: Surface Molecules Escape into the Air
- As energy builds, the fastest-moving molecules on the liquid’s surface break free from the liquid’s surface tension.
- These molecules then enter the air as gas, a process that turns the liquid into vapor.
Step 5: Factors Affecting Evaporation Rate
- Temperature: Higher temperatures speed up evaporation as more molecules have the energy to escape.
- Surface Area: Liquids with a larger surface area (like water spread thinly) evaporate faster.
- Humidity: Lower humidity (dry air) speeds up evaporation because dry air can hold more water vapor.
- Wind: Wind or air movement also helps remove the water vapor, allowing more liquid to evaporate.
Step 6: Evaporation in Everyday Life
Examples: Evaporation helps us cool down when we sweat, as the sweat absorbs heat from our skin to evaporate. Similarly, drying clothes or water from the ocean turning into vapor is all due to evaporation.
Causes of Evaporation
Evaporation is the process where the liquid turns into vapor, typically at the surface. These factors together influence how quickly liquids evaporate in different environments. Here are a few causes of evaporation:
- Temperature: Higher temperatures increase evaporation. Heat provides energy to liquid molecules, making them move faster and escape into the air.
- Surface Area: Liquids with larger exposed surfaces evaporate faster because more molecules are in contact with the air.
- Air Movement: Wind or moving air speeds up evaporation by moving away humid air, allowing more liquid molecules to escape.
- Humidity: Lower humidity promotes evaporation, as dry air can absorb more vapor, while high humidity slows down the process.
- Pressure: Lower atmospheric pressure above a liquid allows molecules to escape more easily, thus increasing evaporation.
Different Features of Evaporation
- Drying Clothes: When we hang wet clothes outside, the water in them evaporates into the air, making the clothes dry.
- Cooling Effect in Sweat: When we sweat, the liquid sweat on our skin evaporates, helping our body cool down on hot days.
- Making Salt from Sea Water: To get salt, people allow seawater to evaporate. When the water disappears, only salt is left behind.
- Cooking and Concentrating Flavors: In cooking, we simmer soups or sauces to evaporate water, which thickens and enhances the flavor.
- Cooling Water in Earthen Pots: In traditional clay pots, water evaporates slowly from the surface, keeping the water inside cool.
- Air Conditioning and Cooling Machines: Some cooling machines use evaporation to cool down air, similar to how our sweat cools us down.
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...
 Reactivity Series of Metals, Charts, Fea...
Reactivity Series of Metals, Charts, Fea...
 Saponification - Define, Reaction, Step ...
Saponification - Define, Reaction, Step ...














