Define Electrolytes
Electrolytes are substances that, when dissolved in a solvent like water, break into ions, which are electrically charged particles. These ions are electrically charged particles. These ions are capable of conducting electricity, facilitating the flow of an electric current through the solution. Common electrolytes include salts, acids, and bases. Electrolytes are integral to various chemical and physical processes, such as electrolysis, where an electric current induces chemical reactions, and batteries, where they help in the movement of charged particles.
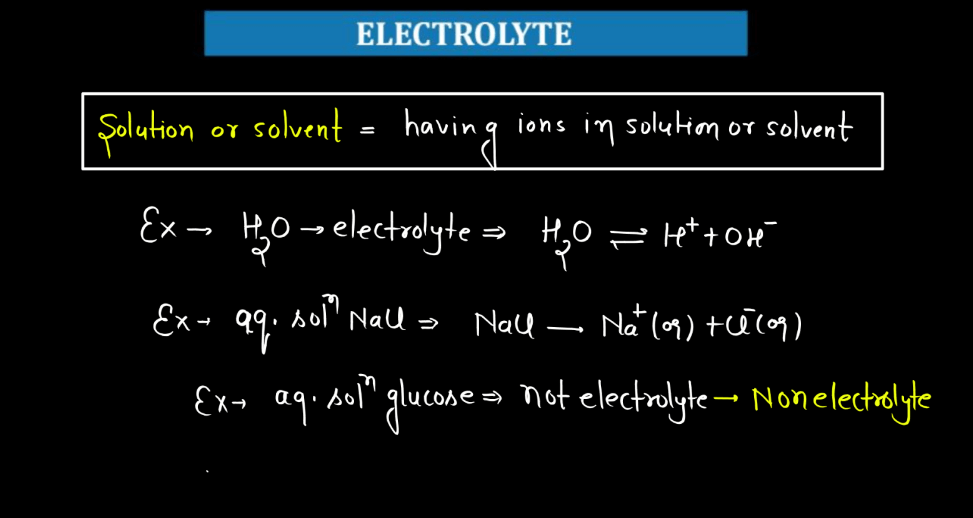
In aqueous solutions, electrolytes can be categorized into strong and weak electrolytes based on their ability to dissociate into ions. In a biological system, electrolytes are crucial for cellular functions, maintaining membrane potentials, and facilitating nerve impulses. Imbalances in electrolyte concentrations can impact cellular processes and lead to health issues. Understanding electrolytes is fundamental in fields ranging from electrochemistry to biochemistry, with applications in industries like medicine, energy, and materials science.
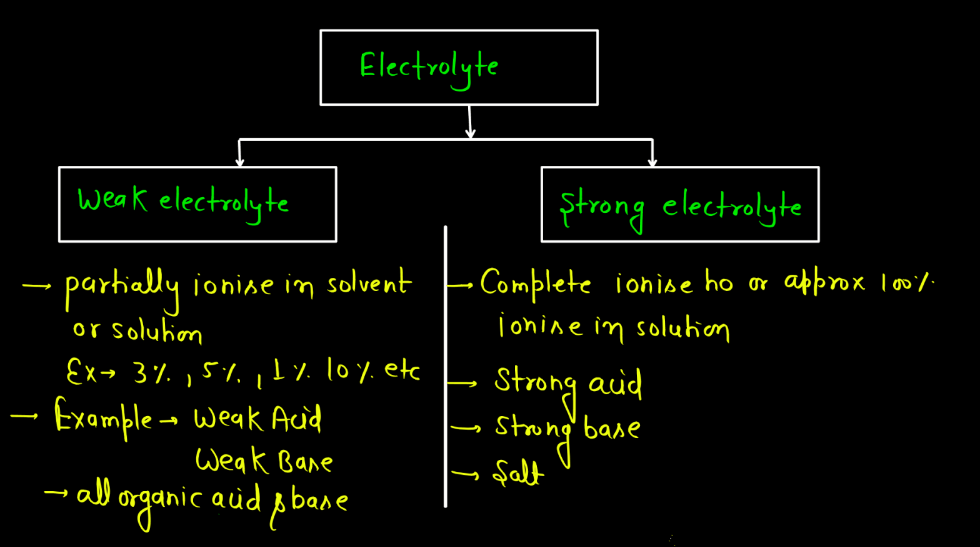
Types of Electrolytes
There are mainly two different types of electrolytes namely: Strong electrolytes and Weak electrolytes. In both cases, electrolytes facilitate the flow of electric current in a solution due to the presence of ions.
| Different Types of Electrolytes | |
| Types of Electrolytes | Description |
| Strong Electrolytes | These substances fully dissociate into ions when dissolved in water, leading to a high conductivity. Examples include salts, strong acids, and strong bases. |
| Weak Electrolytes | These substances only partially dissociate into ions, resulting in lower conductivity. Weak acids and weak bases are examples. They don’t completely break apart in water. |
Define Conductance
Conductance is a measure of how well electricity flows through a material. Think of it like a road for electrons – a high conductance means electrons can easily move through, like a wide and smooth highway, while low conductance is like a narrow, bumpy road. In simple terms, conductance depends on the material. Metals generally have high conductance because their atoms allow electrons to move freely. Insulators, on the other hand, hinder electron flow, leading to low conductance.
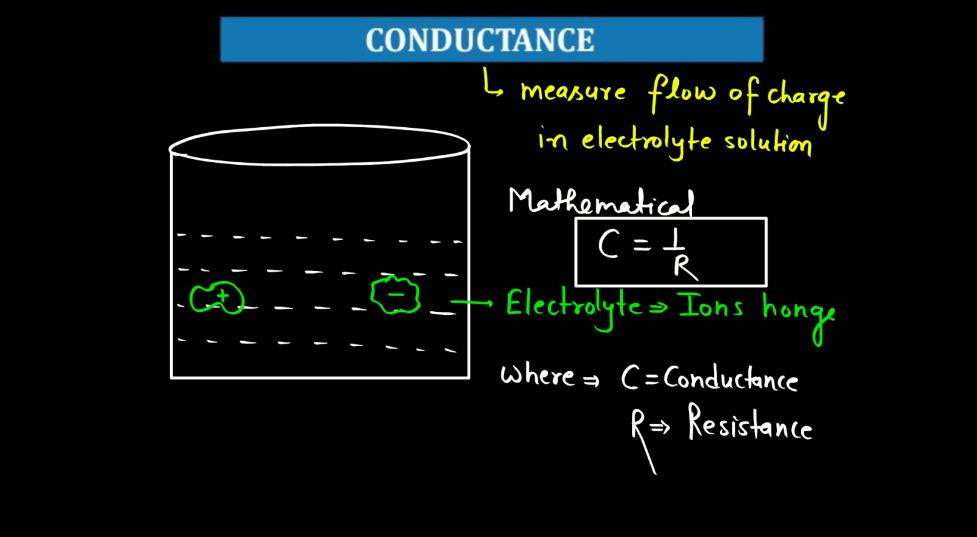
Conductors, like copper, are prized for their high conductance and are commonly used in electrical wiring. It’s essential to distinguish conductance from resistance; they’re inversely related. High conductance means low resistance, and vice versa. You can imagine it as a seesaw – when one side goes up, the other goes down. Understanding conductance helps us design efficient electrical systems, ensuring that electricity flows smoothly where we need it.
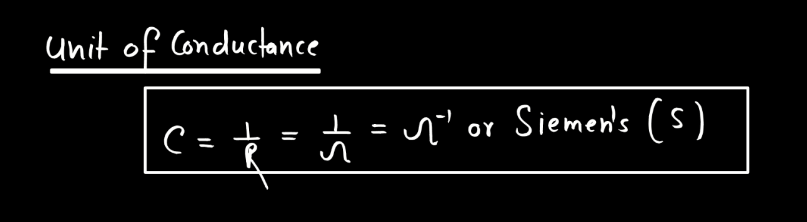
What is Electrolytic Conductance?
Electrolytic conductance refers to the ability of a solution to conduct electricity due to the presence of ions. In simple terms, when certain substances, called electrolytes, dissolve in water, they break into ions – positively charged cations and negatively charged anions. These ions are free to move, carrying an electric charge, allowing the solution to conduct electricity. Imagine it like a team of tiny charged particles, where each ion is like a player with a positive or negative charge.
When you apply an electric field (Voltage) to the solution, these charged players start moving, creating a flow of electricity. Strong electrolytes, like salts, fully dissociate into ions, resulting in higher conductance, while weak electrolytes only partially dissociate. In summary, electrolytic conductance occurs when dissolved ions enable the flow of electricity in a solution, resembling a team of charged players responding to an electric field.
Factors Affecting Electrolytic Conductance
Electrolytic conductance is influenced by factors like the concentration of ions, temperature, and the nature of the electrolyte. Higher ion concentration generally leads to increased conductance, while elevated temperatures enhance conductivity by promoting ion mobility. Additionally, electrolytes with higher dissociation ion mobility. Additionally, electrolytes with higher dissociation into ions tend to exhibit greater conductance.
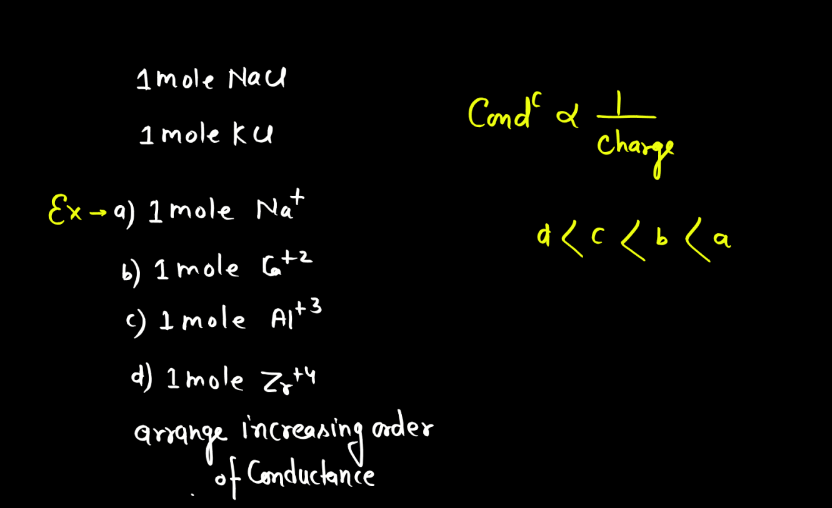
- Nature of the Electrolyte: The nature of electrolytes significantly influences electrolytic conductance. Strong electrolytes, which completely dissociate into ions, exhibit higher conductance than weak electrolytes with partial ion dissociation. The degree of ionization determines the availability of charge carriers, impacting the overall conductivity of the electrolyte solution. Strong acids and bases are examples of strong electrolytes, while weak acids and bases demonstrate lower conductivity due to incomplete dissociation.
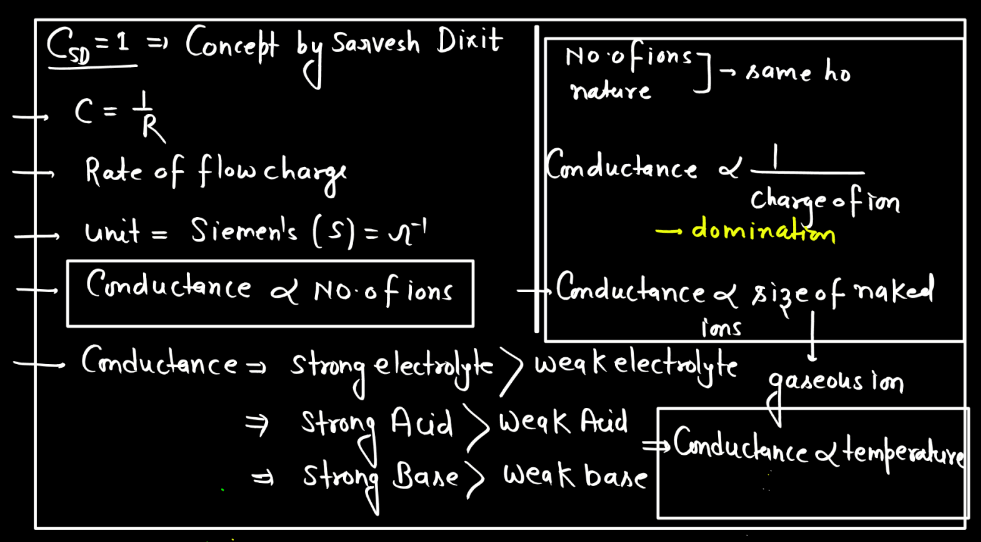
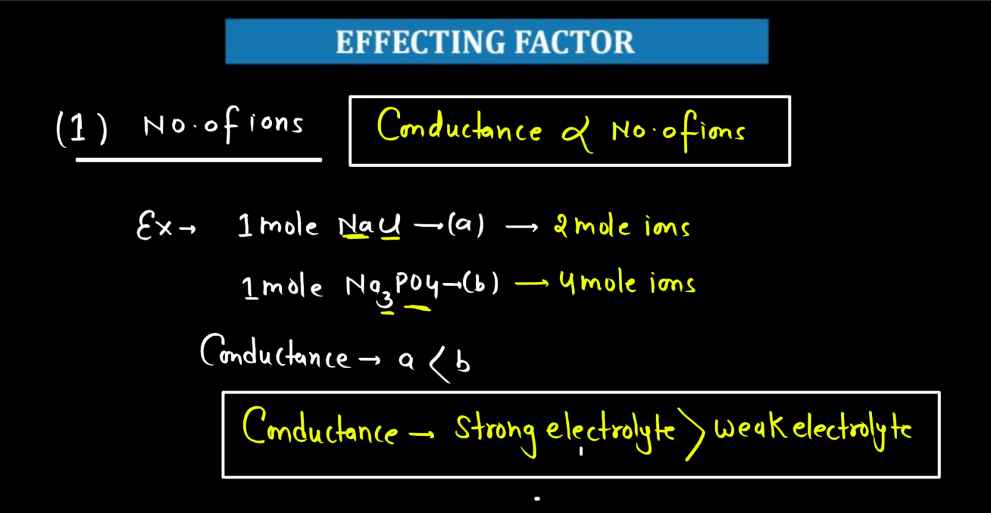
- Concentration of Ions: The higher the concentration of ions in a solution, the better it conducts electricity. More ions mean more charge carriers, facilitating the flow of electric current. Therefore, increased ion concentration generally leads to higher electrolytic conductance in a solution.
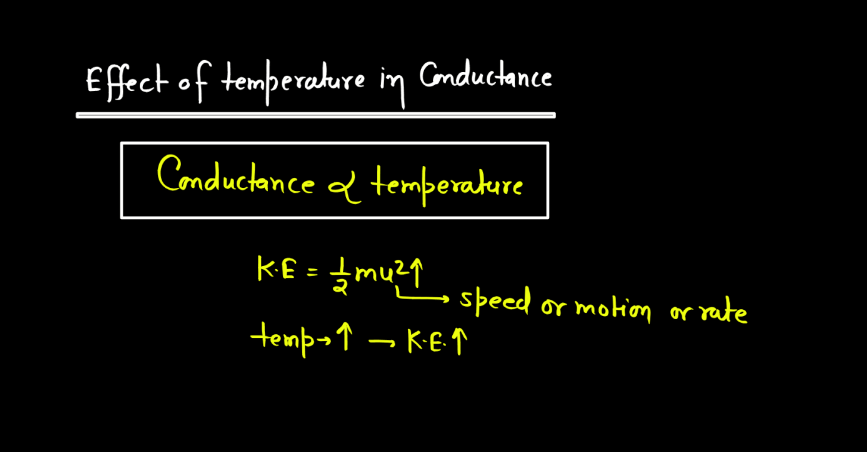
- Temperature: Temperature affects electrolytic conductance by influencing ion mobility. Higher temperatures provide more energy to ions, enabling them to move faster through the solution. This increased mobility enhances electrical conductivity, making electrolytes more effective conductors of electricity at elevated temperatures.
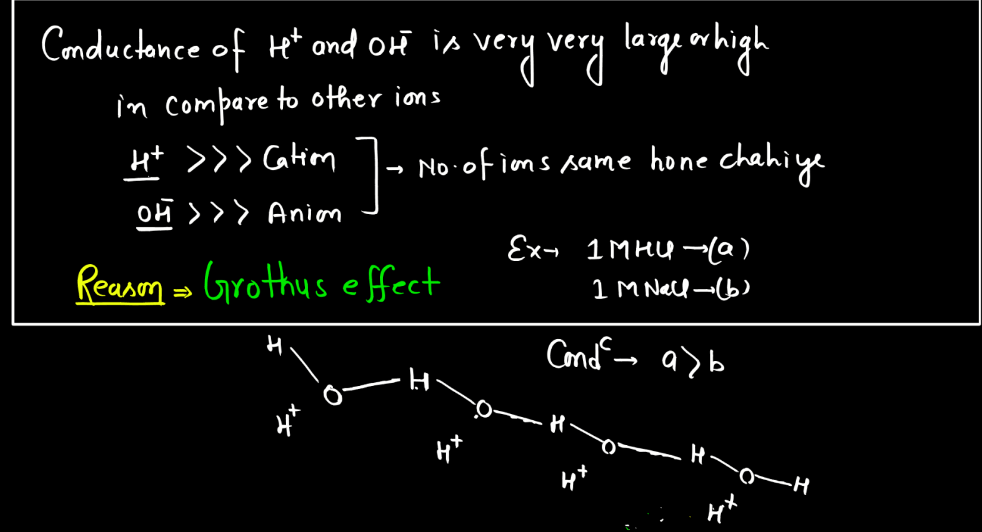
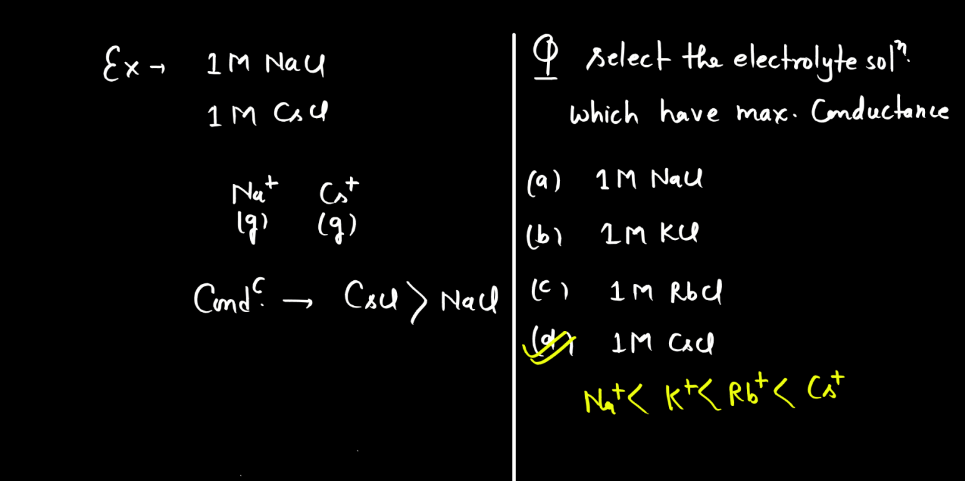
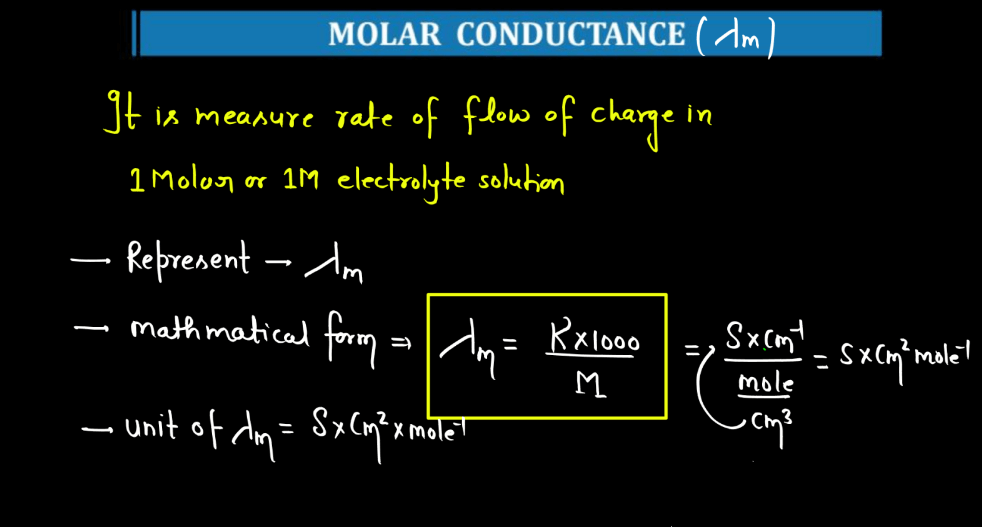
Molar Conductance
Molar conductance is a measure of how well a substance conducts electricity when dissolved in a solution. It quantifies the ability of a given quantity (one mole) of a substance to carry an electrical current between two electrodes in a specific concentration. Molar conductance is often used in the context of electrolytes, substances that ionize into charged particles (ions) when dissolved). The concept is derived from the conductance of ions in solution, where ions facilitate the flow of electric current. Molar conductance accounts for both positive and negative ions, providing a comprehensive measures of the substance’s conductivity. It is expressed in units of siemens per mole square decimeter (Sm²/mol dm³), reflecting the conductivity per unit concentration. This parameter is valuable in understanding and comparing the electrolytic behavior of different substance in solution.
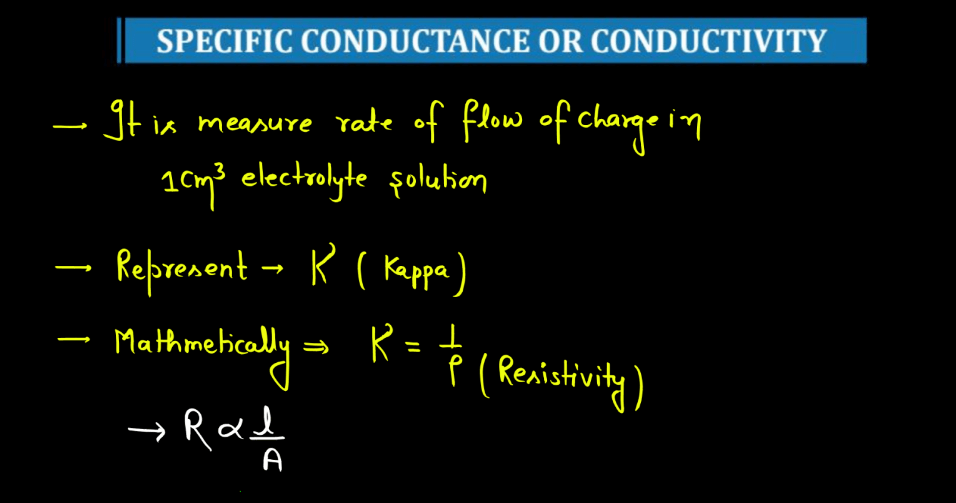
Specific Conductance
Specific conductance, also known as conductivity, measures how well a solution conducts electricity. It is the conductance of a solution between two electrodes placed one unit distance apart, typically in a centimeter or meter. Specific conductance is influenced by the concentration of ions in the solution. In simple terms, when substances dissolve in water, they may form ions (charged particles). The more ions present, the better the solution conducts electricity.
Specicific conductance is expressed in siemens per centimeter (S/cm) or seimens per meter (S/m), representing the conductance of a solution over a specific distance. This property is widely used to assess the purity of water and monitor the concentration of dissolved ions. High specific conductance indicates a greater ability to conduct electricity, often associated with the presence of dissolved salts or other ions in the solution.
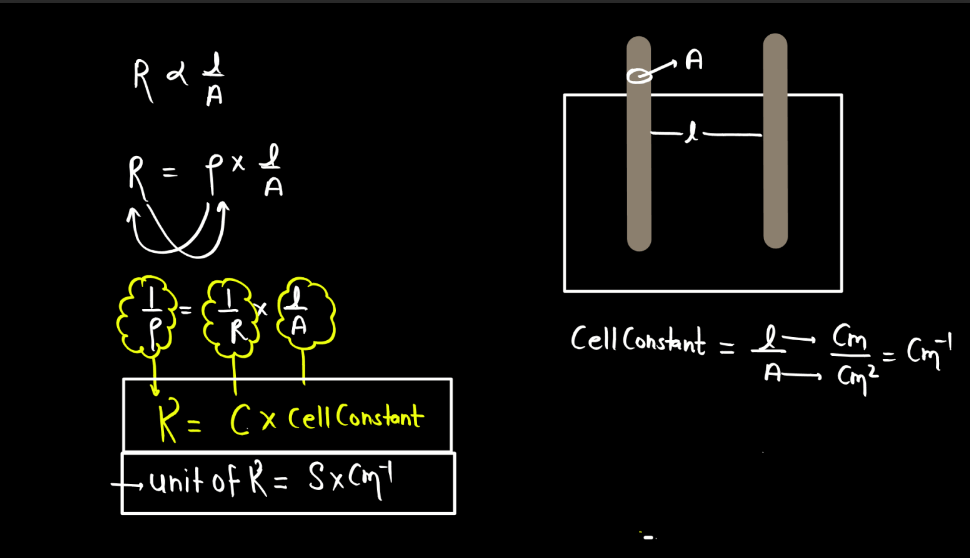
For Detailed Studied About Electrochemistry, Watch the below video:



 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...










