Table of Contents
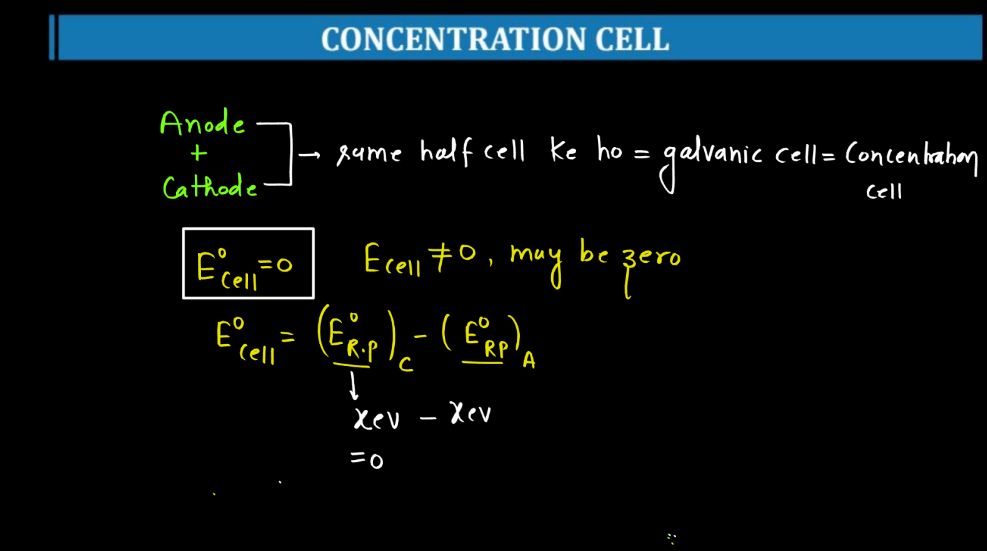
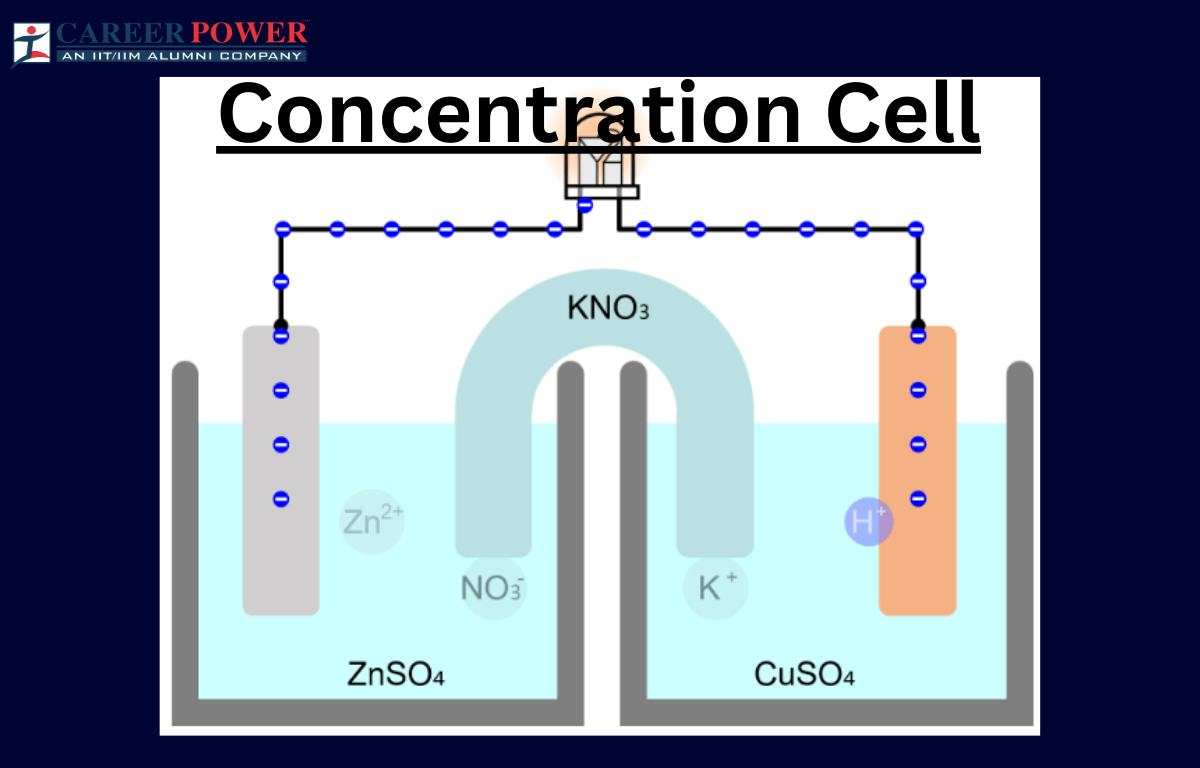
Concentration Cell
A concentration cell is a setup where two half-cells of the same material are connected, but their concentrations differ. Essentially, it’s a battery without different metals. The driving force here is the concentration gradient. For example, if both half-cells contain zinc metal and are immersed in a solution with zinc ions, but one solution is more concentrated than the other, a potential difference arises. This happens because ions move from the more concentrated to the less concentrated side, seeking equilibrium.
This flow of ions generates an electric potential, creating a voltage across the cell. Concentration cells illustrate the impact of concentration charges on electrochemical reactions and help understand how concentration influences cell potential. They are vital in studying electrochemistry and can be found in various applications, including corrosion studies and analytical chemistry.
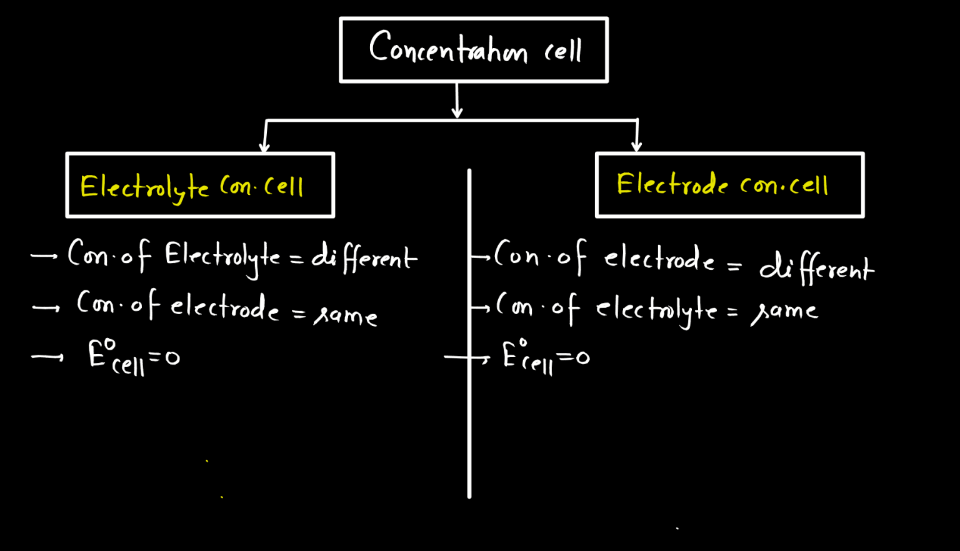
Types of Concentration Cells
Concentration cells are electrochemical cells where the voltage is generated by a difference in concentration rather than different materials. The two types of concentration cells include Electrode Concentration Cell and Electrolyte Concentration Cell.
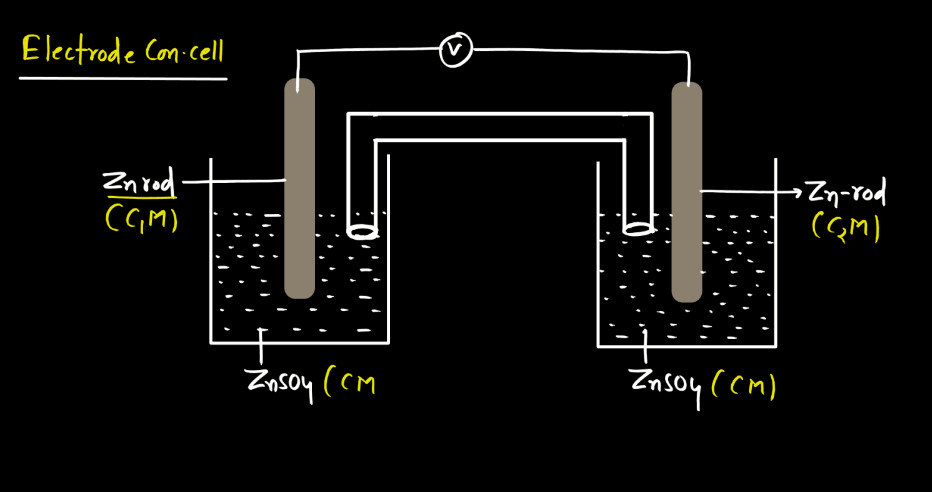
Electrode Concentration Cell
An electrode concentration cell is a setup where two half-cells have the same metal but different ion concentrations. This creates a voltage potential between them, leading to electron flow, like a battery.
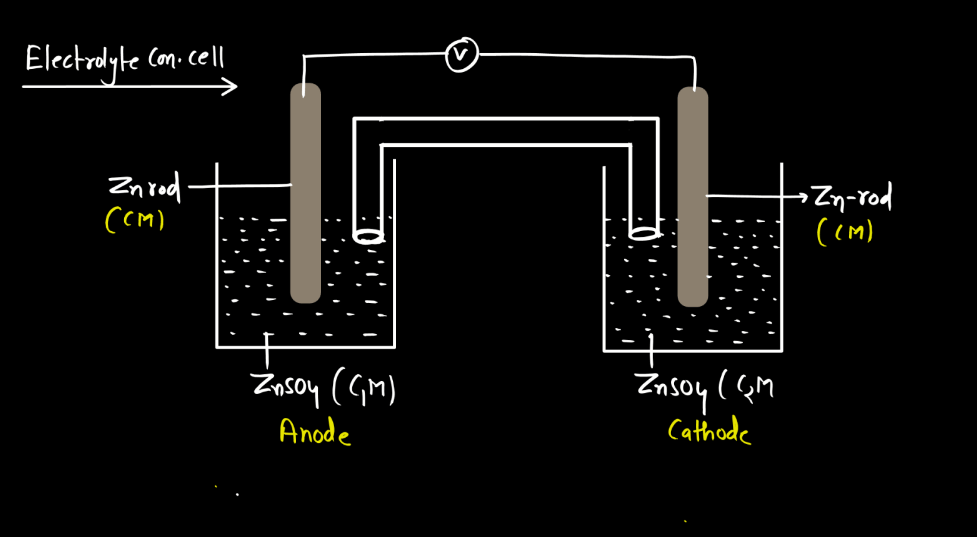
Electrolyte Concentration Cell
An electrolyte concentration cell is a setup with two half-cells having the same substance but different concentrations. It generates electrical potential due to the concentration difference, causing ions to move and creating a cell-like structure.
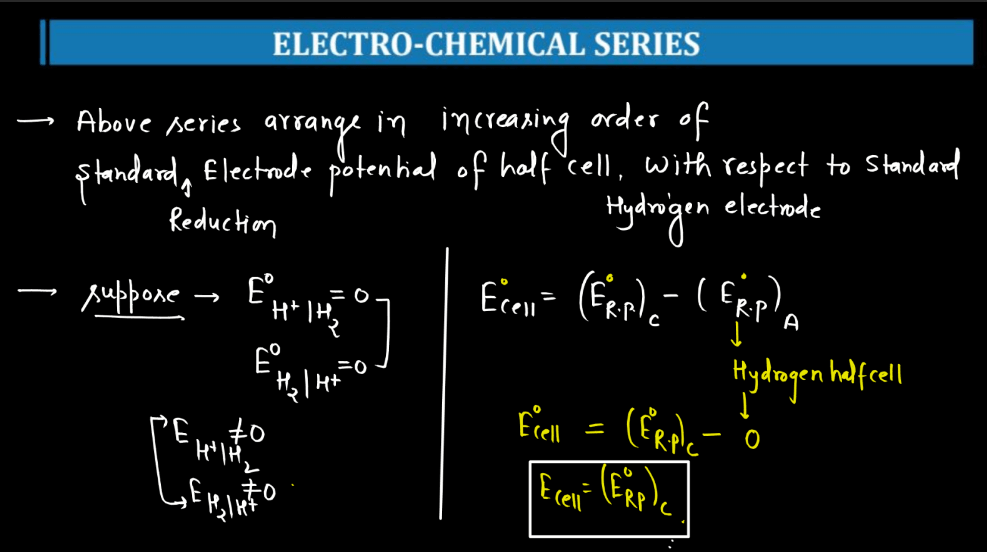
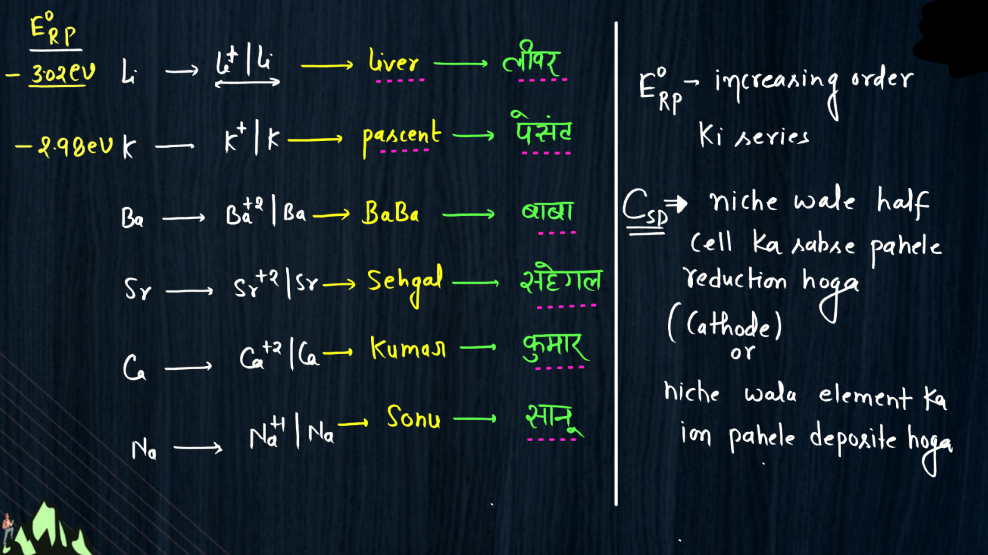
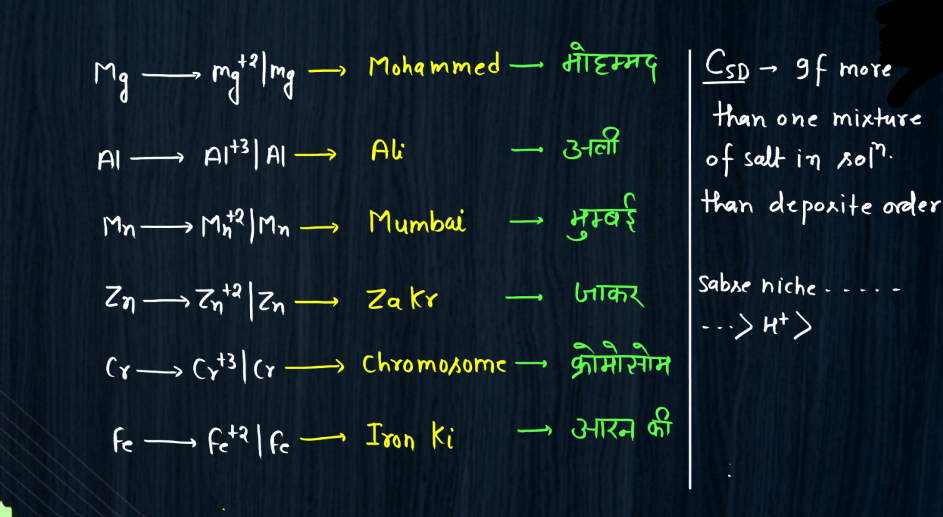
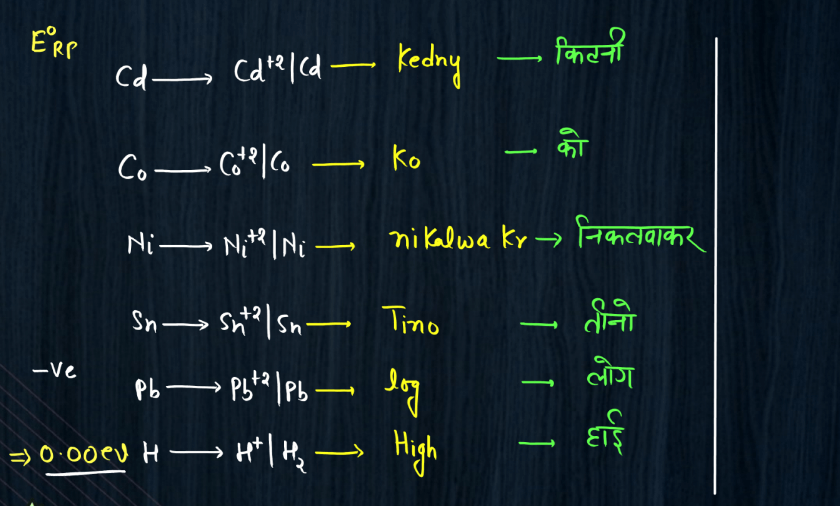
Electro-Chemical Series
The electrochemical series is a ranking of metals and non-metals based on their tendency to gain or lose electrons in chemical reactions. It helps predict the behavior of substances in electrochemical reactions, such as those occurring in batteries. Metals higher in the series are more likely to lose electrons and act as reducing agents, while those lower down tend to gain electrons and act as oxidizing agents. For example, if you have two metals in contact, the one higher in the series will tend to give electrons to the lower one. This flow of electrons creates an electric current, making it the basis for understanding how batteries work. The electrochemical series also illustrates the reactivity of elements, with metals like lithium and sodium being highly reactive and placed at the top, while noble gases like helium and neon, which are less reactive, are found towards the bottom of the series.

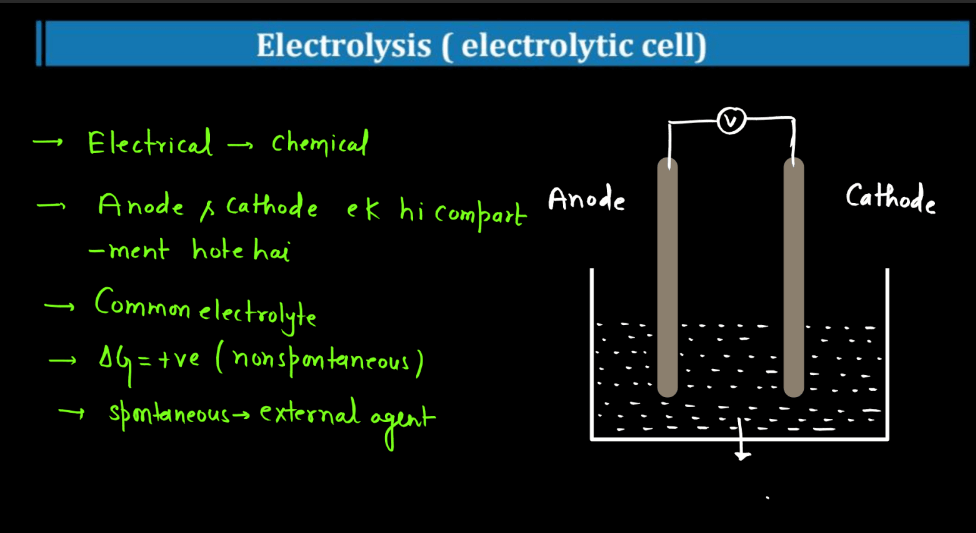
Electrolysis
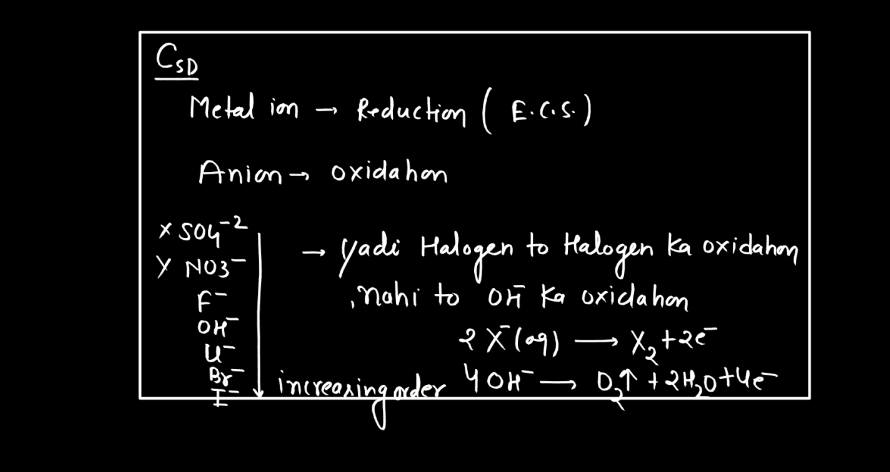
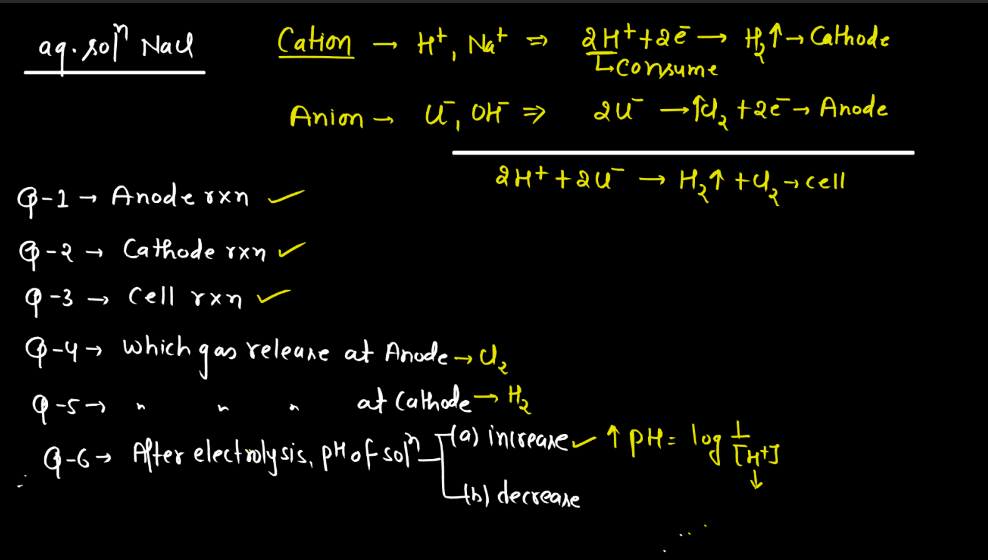
Electrolysis is a process where electrical energy triggers a chemical change in a substance. Imagine a solution with ions (charged particles) dissolved in it. When an electric current is passed through the solution using electrodes (conductors), it causes the ions to move towards the electrodes. At the electrodes, the ions gain or lose electrons, undergoing chemical reactions. For instance, during the electrolysis of water, water molecules split into oxygen and hydrogen gas.
The positive electrode attracts negatively charged ions (anions), while the negative electrode attracts positively charged ions (cations). This separation and rearrangement of ions lead to the formation of new substances. Electrolysis is vital in various applications, from extracting metals from ores to producing gases like hydrogen. It’s a way to harness electrical energy to drive chemical transformations, playing a crucial role in both industrial processes and scientific research.
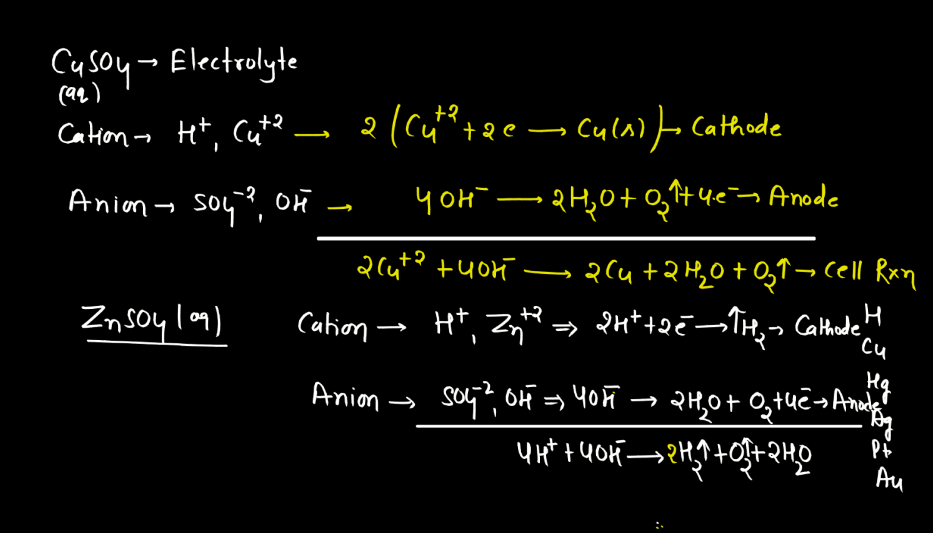
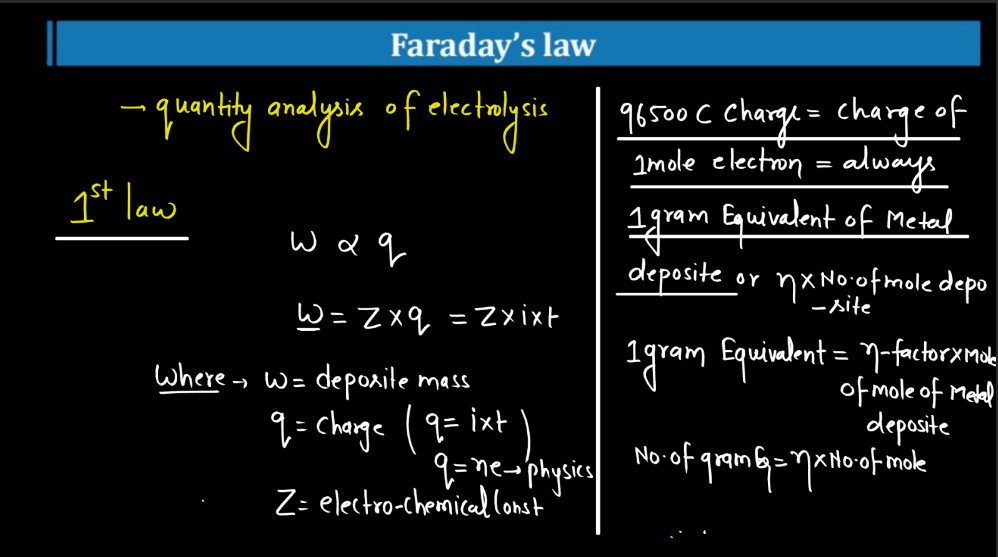
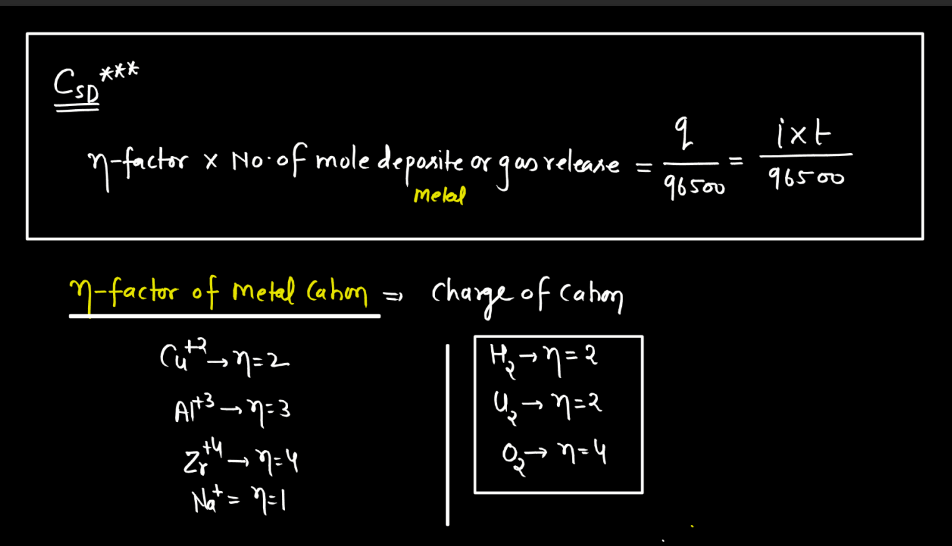
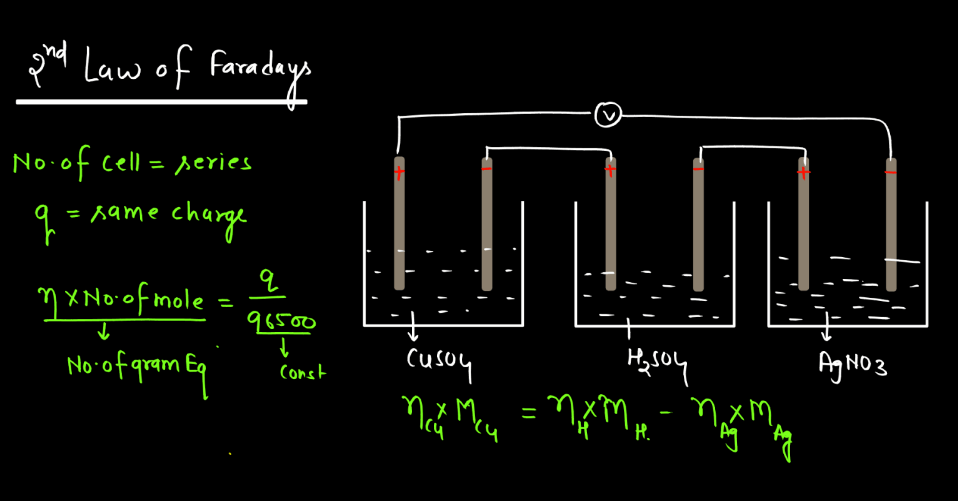
Faraday’s Law
Faraday’s law of electromagnetic induction states that a change in magnetic field within a closed loop induces an electromotive force (EMF) in the loop. The phenomenon, discovered by Michael Faraday, explains how electricity can be generated by moving a magnet near a coil of wire or changing the magnetic field around the coil. The induced EMF is directly proportional to the rate of change of magnetic flux. In simpler terms, when you move a magnet near a wire, it makes electrons in the wire move, creating an electric current. This principle is fundamental to the operation of generation and transmission of electrical energy in various devices and power systems.
| Faraday’s Laws of Electromagnetic Induction | |
| Laws | Description |
| First Law | The induced electromotive force (EMF) in a circuit is directly proportional to the rate of change of magnetic flux through a circuit. |
| Second Law | The magnitude of the induced EMF is equal to the rate of change of magnetic flux through a coil. |
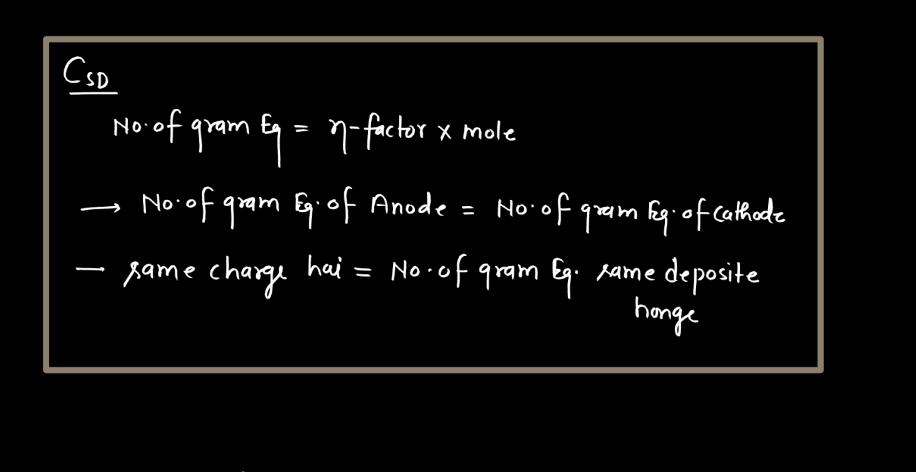
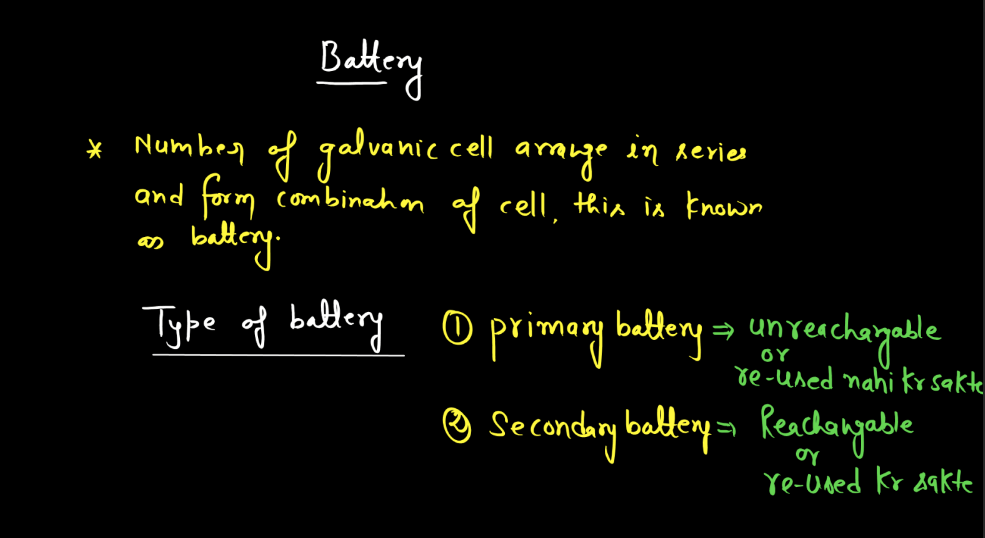
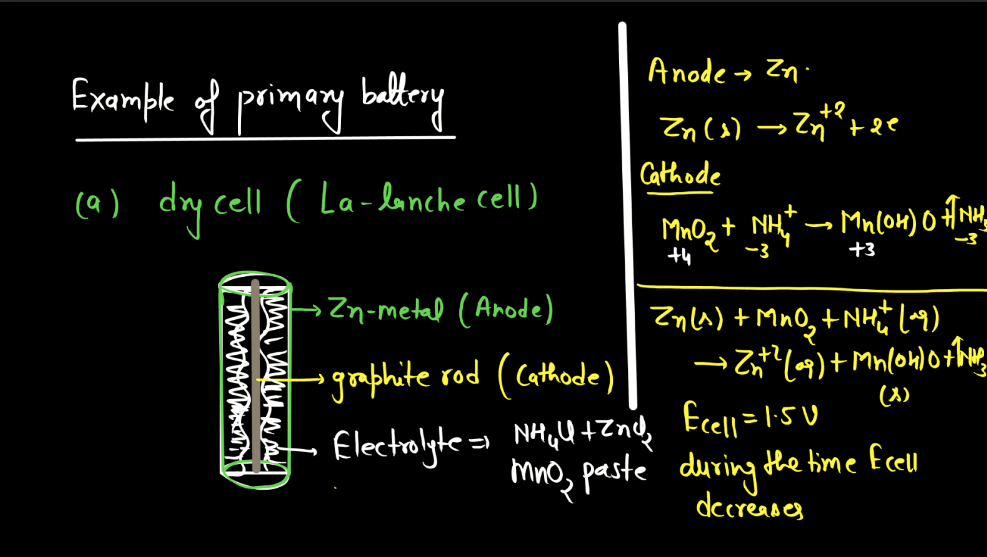
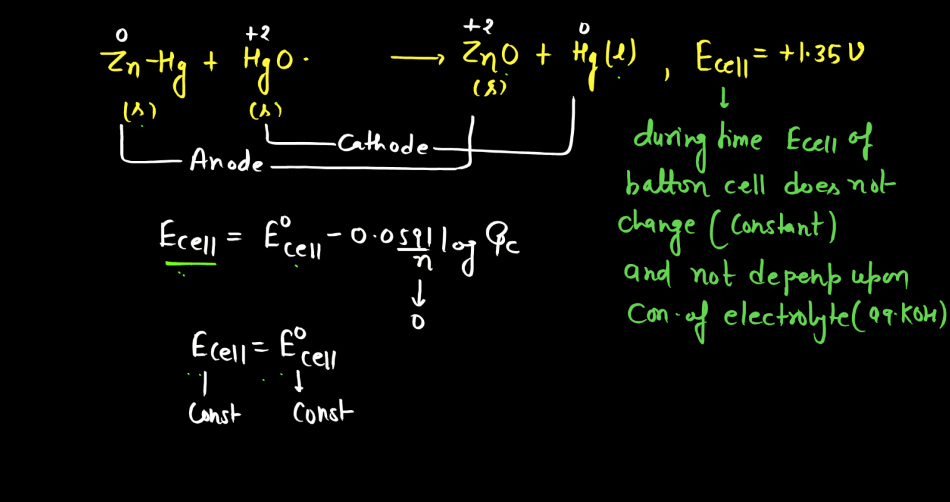
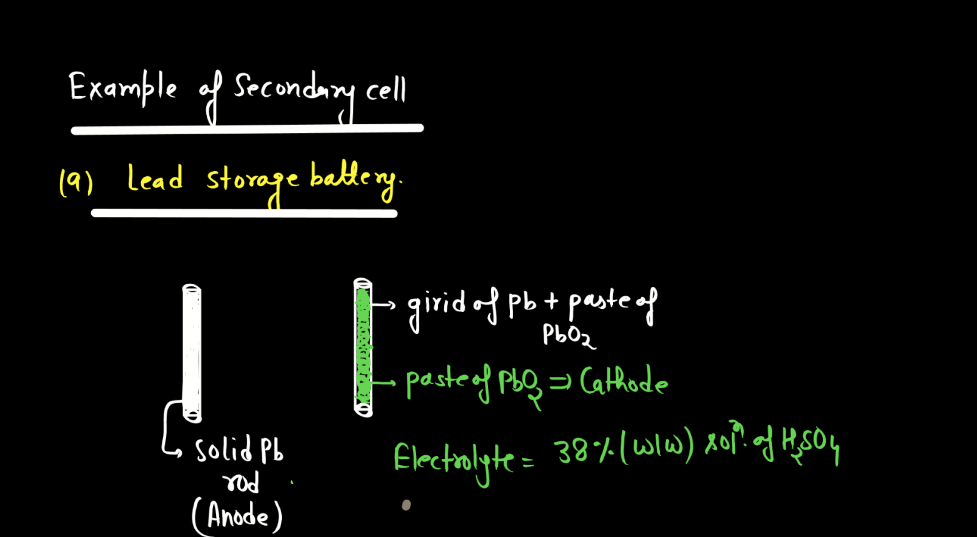
Battery
A battery is a portable energy storage device that converts chemical energy into electrical energy through electromagnetic reactions. Comprising one or more electrochemical cells, a typical battery consists of positive and negative electrodes immersed in an electrolyte. During discharge, electrons flow from the negative to the positive electrode, generating an electric current. Recharging reverses this process. Batteries are essential in powering various electronic devices, from small gadgets to electric vehicles. There are mainly two types of batteries: primary batteries and secondary batteries.
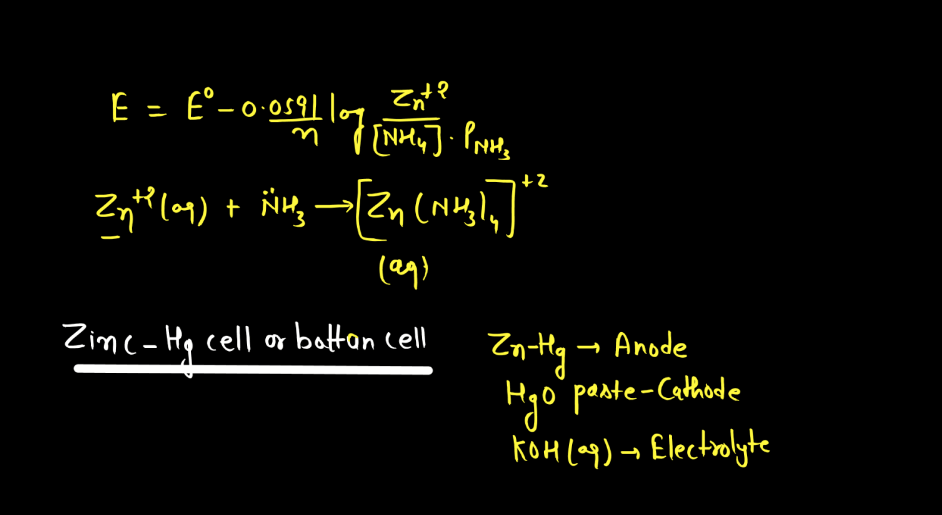
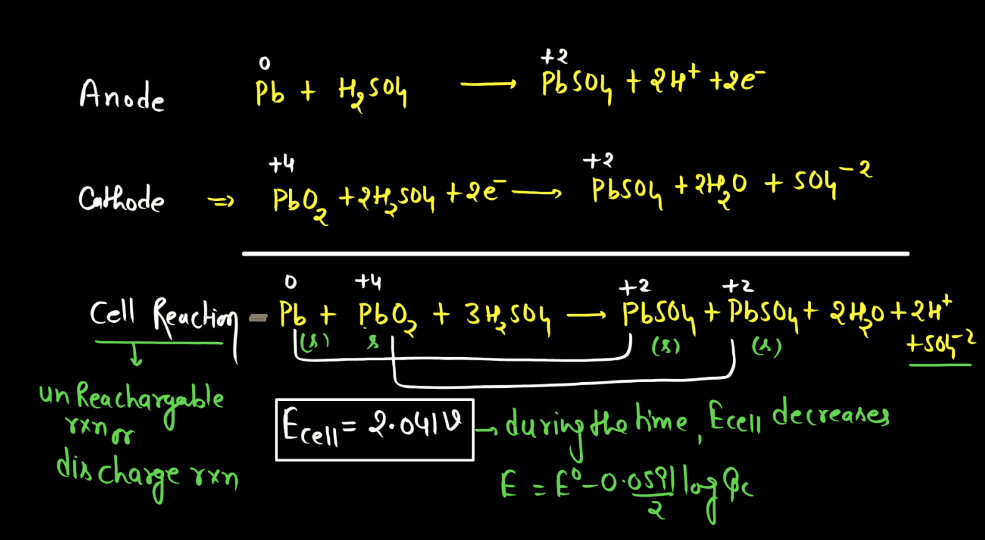
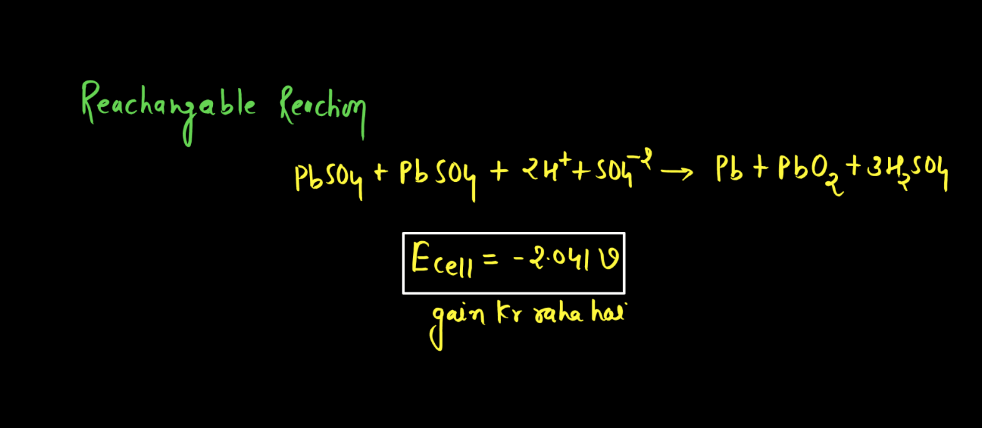
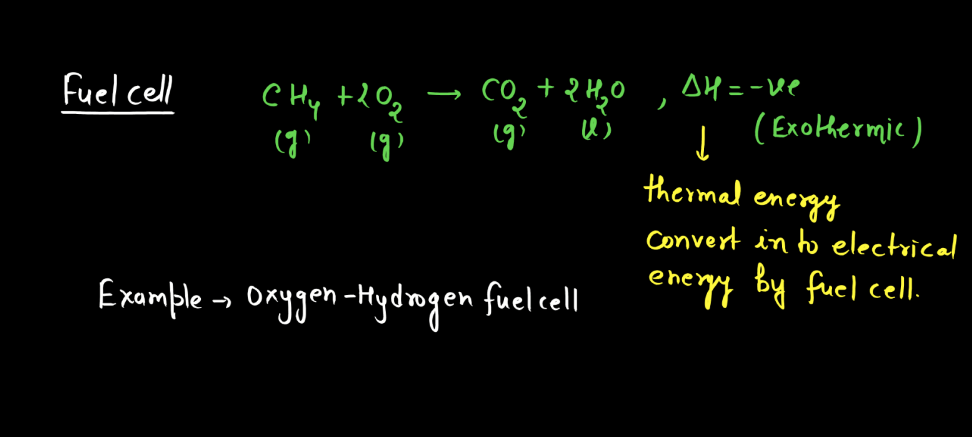
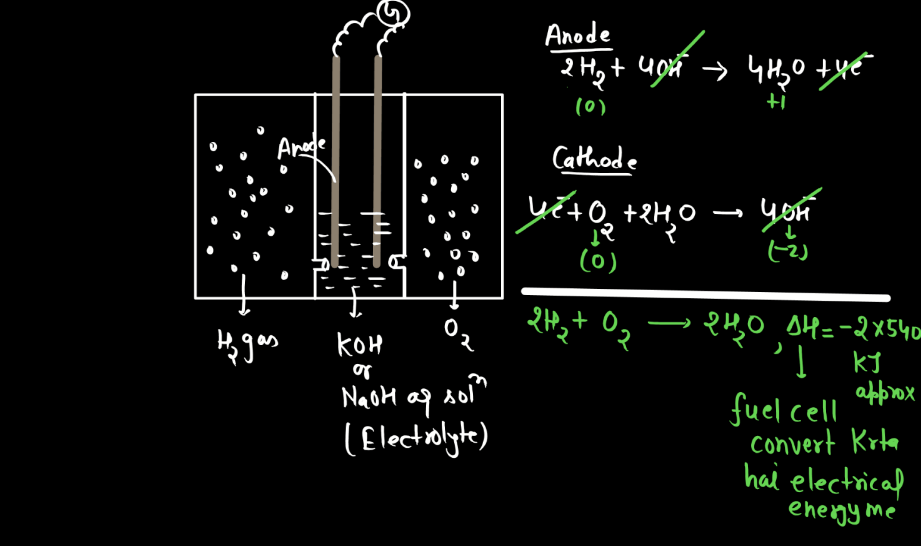
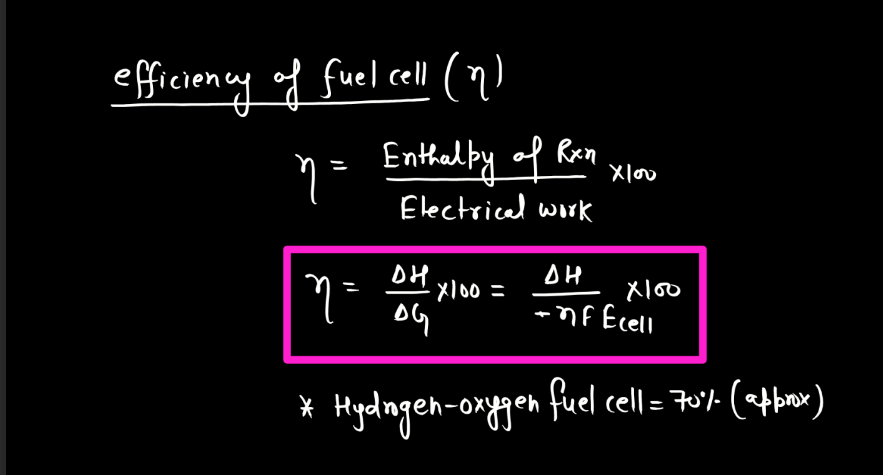
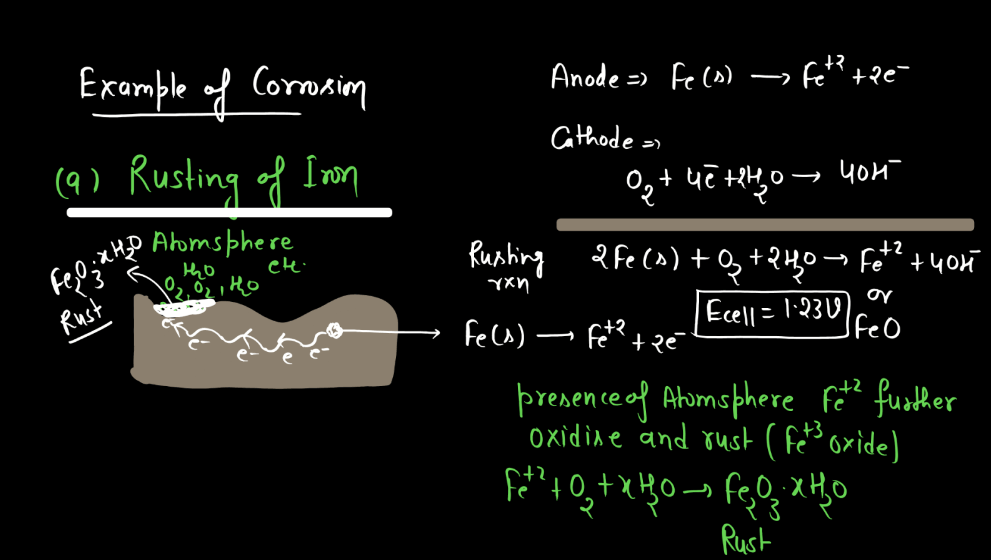
Corrosion
Corrosion refers to the gradual deterioration of materials, typically metals, due to chemical reactions with their environment. This process often involves the metal reacting with substances like oxygen or moisture, forming compounds like rust on iron or tarnish on silver. These reactions weaken the material, leading to structural damage and loss of functionality over time. Corrosion is a natural phenomenon influenced by factors such as humidity, temperature, and the type of metal involved. Preventive measures, like protective coating or alloys, are commonly employed to mitigate corrosion and extend the lifespan of metal objects.

For detailed study, please watch the video given below:








 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...