Table of Contents
As we know, in chemistry reactions are processes where atoms or molecules transform into new substances. These transformations involve the breaking and forming of chemical bonds, resulting in different arrangements of atoms. Here we have briefly discussed the Decomposition Reaction. In a Decomposition reaction, a single compound breaks down into simpler substances. This process happens when the compound is heated or exposed to light.
What is Decomposition Reaction?
A Decomposition reaction is a chemical reaction in which a single compound breaks down into two or more simpler substances. This type of reaction is the opposite of a synthetic reaction. Decomposition reactions are common in various chemical processes and are represented by the general equation AB → A + B. Where AB represents the reactant compound, and A and B represent the products formed after the decomposition. This reaction can be triggered by heat, light, electricity, or the reaction with another substance.
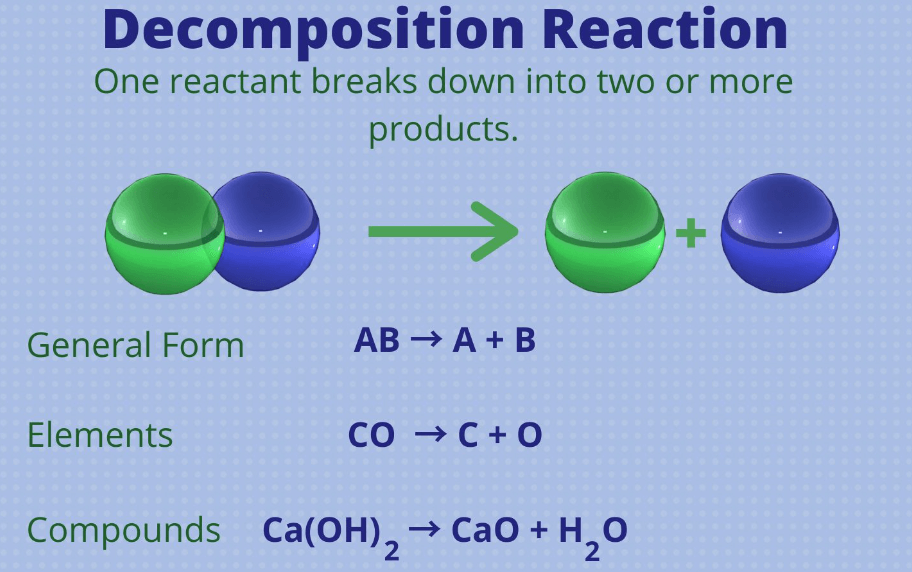
In simple terms, we can say that a decomposition reaction is like breaking a toy into its smaller parts. In chemistry, it’s when a single substance, let’s call it the “toy”, splits apart into smaller pieces. These pieces can be elements or other compounds, just like the toy breaking into smaller parts. For example, think of water (H2O) decomposing into hydrogen gas (H2) and oxygen gas (O2). It is like taking apart a water molecule to get the individual hydrogen and oxygen molecules.
Double Decomposition Reaction
A double decomposition reaction, also known as a double displacement or metathesis reaction, occurs when parts of two reactants exchange places to form new compounds. In these reactions, the cations and anions of two different compounds switch places, resulting in the formation of two entirely different compounds. Here’s a general form of a double decomposition reaction:
AB + CD → AD + CB
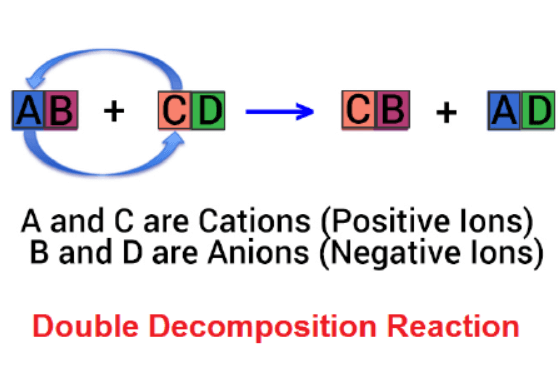
In this reaction, A and C are positive ions (cations), while B and D are negative ions (anions). A and D combined to form one product, while B and C combined to form the other product. A well-known example of a double decomposition reaction is the silver nitrate (AgNO) and sodium chloride (NaCl).
AgNO3 + NaCl AgCl + NaNO3
Type of Decomposition Reaction
There are several types of decomposition reactions based on the way the original substance breaks down. Here we have discussed the three primary types of decomposition reactions:
| Different Types of Decomposition Reactions | ||
| Types of Decomposition Reactions | Description | General Equation |
| Therma Decomposition Reaction | Thermal decomposition occurs when a compound breaks down into simpler substances due to heat energy. | AB → A +B |
| Electrolyte Decomposition Reaction | Electrolytic decomposition happens when a compound breaks down into its elements due to an electric current passing through it. | AB → A + B |
| Photolytic Decomposition Reaction | Photolytic decomposition occurs when a compound breaks down into simpler substances due to light energy. | AB (light) → A + B |
Thermal Decomposition Reaction
The thermal decomposition reaction occurs when a compound breaks down into simpler substances due to heat. For example, the thermal decomposition of calcium carbonate (CaCO3) into calcium oxide (CaO) and carbon dioxide (CO2) when heated.
CaCO3 (Solid) → CaO (Solid) + CO2 (Gas)
Electrolytic Decomposition Reaction
This type of decomposition happens when an electric current passes through a compound, causing it to break down into its elements. For example, the electrolysis of water (H2O) produces hydrogen gas (H2) and oxygen gas (O2).
2H2O (Liquid) → 2H2 (Gas) + O2 (Gas)
Photolytic Decomposition Reaction
In a photolytic decomposition reaction, a compound breaks down into simpler substances due to light energy. For instance, the decomposition of silver chloride (AgCl) into silver (Ag) and chlorine gas (Cl2) in the presence of light.
2AgCl (Solid) + Light → 2Ag (Solid) + Cl2 (Gas)
Examples of Decomposition Reaction
Decomposition reaction involve the breakdown of a compound into a simpler substance. Below we have discussed a few examples of decomposition reactions, where a single compound breaks down into two or more simpler substances.
- Hydrogen Peroxide Decomposition: In this reaction, hydrogen peroxide breaks into water and oxygen gas.
2H2O2 → 2H2O + O2
- Calcium Carbonate Decomposition: In this reaction, calcium carbonate decomposes into calcium oxide and carbon dioxide gas.
CaCO3 → CaO + CO2
- Water Decomposition: Here water decomposes into hydrogen gas and oxygen gas, usually through electrolysis.
2H2O → 2H2 + O2
- Ammonium Nitrate decomposition: In this reaction, the ammonium nitrate decomposes into nitrous oxide and water.
NH4NO3 → N2O + 2H2O
- Potassium Chlorate Decomposition: Here potassium chlorate decomposes into potassium chloride and oxygen.
2KClO3 2KCl + 3O2
Uses of Decomposition Reaction
Decomposition reactions have various practical applications in different fields. We have discussed a few examples of decomposition reactions below that have practical applications in diverse fields, ranging from chemistry and industry to everyday products and processes.
- Prevention of Food: Sodium nitrite (NaNO2) decomposes and forms nitric oxide, which is used as a preservative and color fixative in processed meats.
- Fertilizer Production: Ammonium nitrate decomposes to form nitrous oxide and water. Nitrous oxide is used in some fertilizers.
- Chemical Analysis: Decomposition reactions are used in chemical analysis techniques to identify and quality compounds. For example, analyzing the products of a decomposition reaction can help determine the composition of the original compound.
- Hydrogen Production: Water can be decomposed into hydrogen gas and oxygen gas through electrolysis, a process used to produce hydrogen for fuel cells and other industrial applications.
- Oxygen Production: The decomposition of hydrogen peroxide produces oxygen gas, which is used in various applications, including life support systems in spacecraft and hospitals.







 Modes of Heat Transfer with Examples
Modes of Heat Transfer with Examples
 Evaporation - Definition, Step-Wise Proc...
Evaporation - Definition, Step-Wise Proc...
 What is Sedimentation, Decantation and F...
What is Sedimentation, Decantation and F...