Table of Contents
The ozone layer in Biology can be thought of as a natural shield high in the Earth’s atmosphere made of Ozone (O3) molecules. In simple words ozone layer is like Earth’s sunscreen, shielding us from the sun’s harmful ultraviolet rays. It is found in the Earth’s Stratosphere. Ozone layer depletion is when this protective layer gets thinner because of chemicals we release into the air, making more harmful sunlight reach us. This can lead to problems like skin cancer and harm to nature. The World Ozone Day is celebrated on 16th September every year to raise awareness about ozone layer protection.
What is an Ozone Layer?
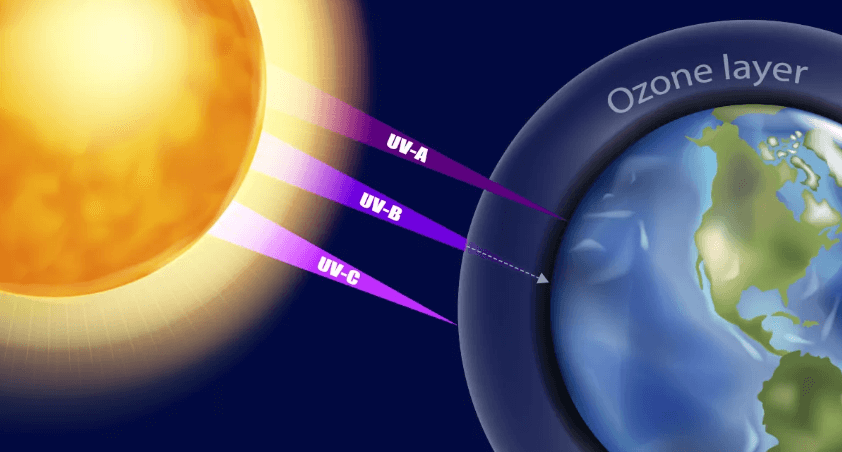
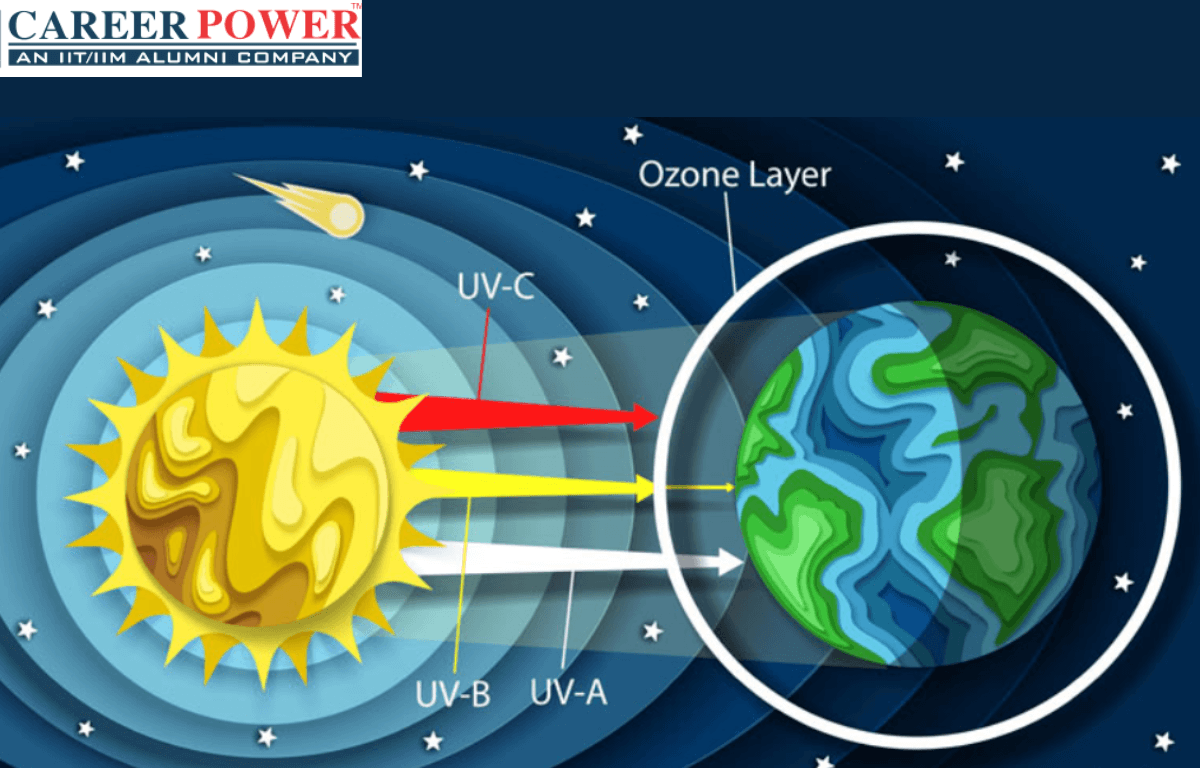
The Ozone layer is a region of Earth’s stratosphere that contains a higher concentration of ozone (O3) molecules compared to other parts of the atmosphere. It plays a crucial role in protecting life on Earth by absorbing and blocking most of the sun’s harmful ultraviolet (UV) radiation. This layer helps prevent excessive UV radiation from reaching the Earth’s surface, which can cause skin cancer, cataracts, and other health issues, as well as damage ecosystems. Efforts have been made to reduce the release of ozone-depleting substances, such as chlorofluorocarbons (CFCs), to help repair and preserve the ozone layer. There are 5 layers of the atmosphere and the ozone layer is the part of Stratosphere.
Some More Information on the Ozone Layer
Now let us discuss a few more points that will help you understand the Ozone Layer more properly.
- Location: The ozone layer is primarily located in the lower portion of the Earth’s stratosphere, approximately 10 to 30 kilometers (6 to 19 miles) above the Earth’s surface.
- Ozone Formation: Ozone is formed naturally through the interaction of solar ultraviolet (UV) radiation with oxygen molecules (O2). This process results in the creation of ozone molecules (O3).
- Ozone Depletion: Human activities, especially the release of ozone-depleting substances like CFCs and halons, have led to the thinning of the ozone layer in certain regions, particularly over Antarctica. This phenomenon is often referred to as the “Ozone Hole”.
- Consequences of Ozone Depletion: Depletion of the ozone layer allows more harmful UV-B and UV-C radiation to reach the Earth’s surface, leading to increased risks of skin cancer, cataracts, and other health problems. It can also harm marine ecosystems, disrupt food chains, and affect terrestrial plant life.
- Ozone Layer Recovery: Due to the efforts made under the Montreal Protocol, there have been signs of recovery in the Ozone Layer. Scientists expect the ozone layer to gradually heal over the coming decades.
- Monitoring: Ongoing monitoring of the ozone layer’s thickness and composition is carried out using satellite-based instruments and ground-based observations to ensure its protection.
Ozone Layer Depletion
Ozone Layer depletion refers to the gradual thinning of the ozone layer in Earth’s stratosphere, particularly in the ozone layer’s lower region known as the ozone hole. This depletion is primarily caused by human-made chemicals called ozone-depleting substances (ODS), such as chlorofluorocarbons (CFCs) and halons. These substances release chlorine and bromine atoms when they break down in the atmosphere, which then destroy ozone molecules.
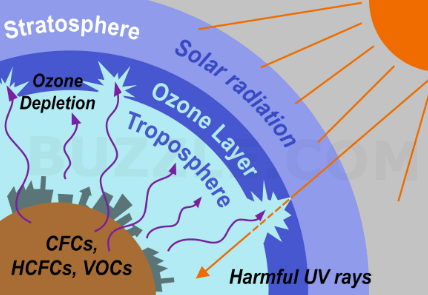
The ozone layer plays a crucial role in protecting life on Earth by absorbing and blocking most of the harmful ultraviolet (UV) radiation from the sun. Ozone depletion leads to an increase in UV radiation at the Earth’s surface, which can have harmful effects on human health, ecosystems, and the environment, including an increased risk of skin cancer, cataracts, and damage to marine life.
Efforts to address ozone layer depletion have been largely successful, thanks to international agreements like the Montreal Protocol. This treaty has phased out the production and use of ODS, leading to signs of ozone layer recovery. However, continued vigilance and adherence to these agreements are necessary to ensure the long-term recovery and protection of the ozone layer.
Substances Responsible for Ozone Depletion
Here we have discussed a few substances that were commonly used in various industrial and consumer applications, but their production and use have been regulated and reduced over time to mitigate ozone layer depletion, primarily through international agreements like the Montreal Protocol.
| Substances responsible for Ozone Depletion | |
| Ozone-Depleting Substances | Common Sources |
| Chlorofluorocarbons (CFCs) | Air conditioning, refrigeration, aerosol sprays, foam insulation, and some solvents. |
| Halons | Fire extinguishers, specialized fire suppression system. |
| Carbon Tertrachloride (CCl4) | Used in the production of CFCs and as a solvent for various industrial processes. |
| Methyl Chloroform (CH3CCl3) | Used as a solvent, particularly in the electronics industry. |
| Hydrochlorofluorocarbon (HCFCs) | Air conditioning, refrigeration, and foam-blowing agents (as transitional replacements for CFCs). |
| Hydrobromofluorocarbon (HBFCs) | Fire extinguishing, chemical manufacturing, and other industrial applications. |
Causes of Ozone Layer Depletion
Ozone depletion is primarily caused by the release of human-made chemicals known as ozone-depleting substances (ODS). Ozone-depleting substances release chlorine and bromine atoms into the stratosphere, where they catalytically break down ozone molecules, leading to the thinning of the ozone layer. The main ODS responsible for ozone layer depletion include:
- Chlorofluorocarbons (CFCs): CFCs are synthetic compounds used in refrigeration, air conditioning, aerosol propellants, and foam-blowing agents. When released into the atmosphere, CFC molecules eventually reach the stratosphere, where they are broken down by ultraviolet (UV) radiations, releasing chlorine atoms that catalytically destroy ozone molecules.
- Halons: Halons are similar to CFCs and are often used in fire extinguishers. They contain bromine and chlorine, both of which contribute to ozone depletion when released into the atmosphere.
- Carbon Tetrachloride (CCl4): This chemical was used in the production of CFCs and as a solvent for various industrial processes. It releases chlorine when it breaks down in the stratosphere.
- Hydrochlorofluorocarbons (HCFCs): HCFCs are used as replacements for CFCs because they have lower ozone-depleting potential. However, they still have some ozone-depleting potential, and their production and use are being phased out under the Montreal Protocol.
- Hydrobromofluorocarbons (HBFCs): Similar to HCFCs, HBFCs contain bromine and chlorine and have ozone-depleting potential. They are also being phased out.
Effects of Ozone Layer Depletion
Ozone layer depletion has significant and far-reaching effects on the environment, human health, and ecosystems. Efforts to reduce ozone-depleting substances (ODS), primarily through international agreements like the Montreal Protocol, have been successful in halting and reversing ozone layer depletion. However, it will still take several decades for the ozone layer to fully recover, and continued vigilance is essential to protect this crucial layer of our atmosphere.
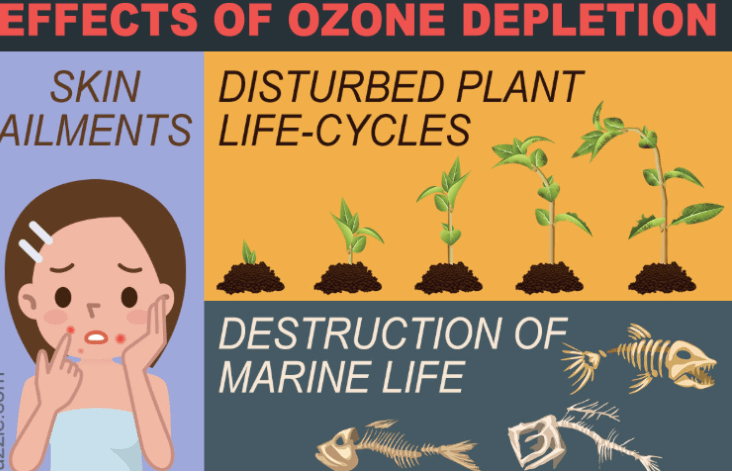
- Increased Ultraviolet (UV) Radiation: Ozone in the stratosphere absorbs and blocks a significant portion of the sun’s harmful ultraviolet (UV) radiation. Depletion of the ozone layer leads to higher levels of UV radiation reaching the Earth’s surface.
- Skin Cancer: Increased UV radiation is a major risk factor for skin cancer in humans. Prolonged exposure to UV rays can lead to the development of skin cancers, including melanoma, squamous cell carcinoma, and basal cell carcinoma.
- Eye Damage: UV radiation can also cause eye damage, including cataracts and other eye conditions that can impair vision.
- Crop Damage: Increased UV radiation can harm crops, reducing agricultural yields and affecting food security.
- Harm to Marine Life: UV radiation can penetrate the ocean’s surface and harm marine ecosystems. It can damage phytoplankton, which forms the basis of the marine Food chain, and harm coral reefs.
- Climate Change: Changes in UV radiation can influence atmospheric circulation patterns, potentially impacting weather and climate systems.







 50 Vegetables Name for Kids in English a...
50 Vegetables Name for Kids in English a...
 Body Parts Name, All 50 Body Parts Name ...
Body Parts Name, All 50 Body Parts Name ...
 Flowers Names in English and Hindi, List...
Flowers Names in English and Hindi, List...